Cignal Reporter Assay Kits
For rapid, sensitive, and quantitative assessment of signal transduction pathway activation
For rapid, sensitive, and quantitative assessment of signal transduction pathway activation
Cat. No. / ID: 336841
Cignal Reporter Assays provided in the Cignal Reporter Assay Kit enable rapid, sensitive, and quantitative assessment of signal transduction pathway activation by measuring the activities of downstream transcription factors. Two reporter systems are available: dual-luciferase format and GFP format.
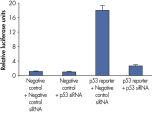 Assessing overexpression phenotypes.
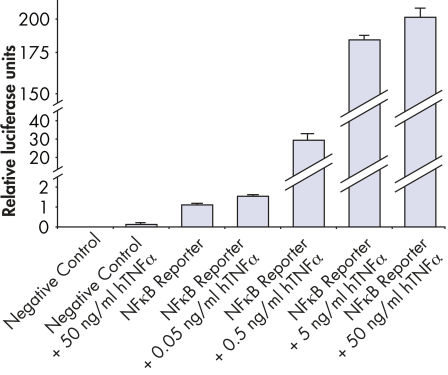
Assessing overexpression phenotypes.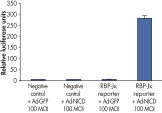 TNFα activates NFkB signaling activity in a dose-dependent manner.
TNFα activates NFkB signaling activity in a dose-dependent manner.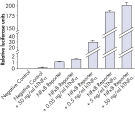 Verification of small molecule drug candidate.
Verification of small molecule drug candidate.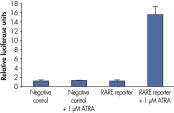 Cignal Reporter Assay shows that hTNFa activated NFkB signaling pathway.
Cignal Reporter Assay shows that hTNFa activated NFkB signaling pathway.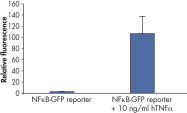 Cignal Reporter Assay measures activation of serum response factor (SRF) transcription activity.
Cignal Reporter Assay measures activation of serum response factor (SRF) transcription activity.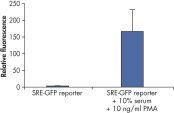
Cignal Reporter Assays consist of multiple repeats of a specific transcription factor’s binding site and basic promoter elements to drive the expression of a reporter gene (firefly luciferase or GFP). These reporters are powerful tools in functional genomics and drug discovery for assessing pathway activity. When the pathway is activated or inhibited by a drug candidate, gene knockdown, overexpression, or peptide, luciferase or GFP reporter activity is modulated and can be measured quantitatively and rapidly (see figure " Cignal Reporter Assay principle"). The Cignal Reporter Assays are available as transfection-ready constructs utilizing two reporter systems: the dual-luciferase reporter or the GFP reporter (see flowchart " Cignal Reporter Assay procedure").
The dual-luciferase format is an end-point assay providing unsurpassed sensitivity, specificity, and signal-to-noise ratios. This format is available for all Cignal Reporter Assays. Each assay includes a pathway-focused transcription factor reporter and a noninducible negative control, as well as luciferase and GFP positive controls.
The GFP format is an outstanding method for monitoring live cell pathway regulation, with single cell resolution. It includes a pathway-focused transcription factor reporter and positive and negative controls. >
Cignal Reporter Assays include a preformulated, transfection-ready pathway reporter (dual-luciferase or GFP), plus a positive and negative control. The inducible pathway reporter and noninducible negative control are transfected and subjected to experimental treatments in parallel.
Dual-luciferase results are calculated for each transfectant. The change in the activity of each signaling pathway is determined by comparing the normalized luciferase activities of the reporter in treated versus untreated transfectants. The identically treated negative control transfectants serve as a specificity control. The positive control serves as a control for transfection efficiency, by monitoring firefly luciferase and Renilla expression.
GFP expression is quantified using a flow cytometer, fluorescence microscope, or fluorometer. The change in the activity of each signaling pathway is determined by comparing the GFP activities in treated versus untreated transfectants. The identically treated negative control transfectants serve as a specificity control. The positive control serves as a control for transfection efficiency, by monitoring GFP expression from the constitutively expressing CMV-GFP reporter. >
Cignal Reporter Assays are powerful tools in functional genomics, proteomics, and drug discovery for assessing the biological impact of pathway inhibition or activation with siRNAs, proteins, and small molecule compounds.