QIAGEN Large-Construct Kit
最大50 μg BAC、PAC、P1 DNAすなわち最大200 μgコスミドDNA、不含ゲノムDNAの精製用
最大50 μg BAC、PAC、P1 DNAすなわち最大200 μgコスミドDNA、不含ゲノムDNAの精製用
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
カタログ番号 / ID. 12462
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
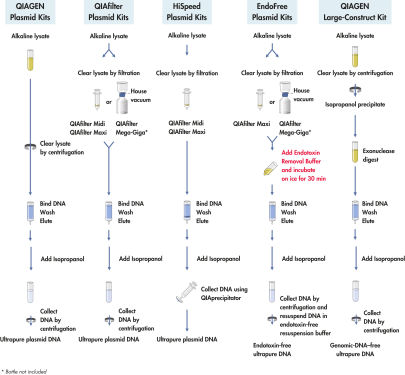
QIAGEN Large-Construct Kitは、高分子量DNAの精製用、自然落下式の陰イオン交換型カラムを提供します。独自の統合型ATP依存性エクソヌクレアーゼ消化ステップにより、混入ゲノムDNAが選択的に除去できるようになります。精製されたDNAは、CsCl密度勾配遠心法を2回行なって得られる純度に匹敵し、トランスフェクションに適しています。
QIAGEN Large-Construct Kitを用いるDNA精製では、最適化した自然落下法による操作手順を用い、これにより、他の一般的な方法と比較して大幅に純度の高いDNAが得られます。ATP依存性エキソヌクレアーゼを組み込んだ独自の処理により、ゲノムDNAを効率的に除去できます。
QIAGEN Large-Construct Kitに付属のQIAGEN-tips内にある独自の陰イオン交換樹脂は、核酸の精製専用に開発されています。本製品の優れた核酸分離能力により、CsCl密度勾配遠心法を2回連続で行なって得られるDNA純度に匹敵するかそれ以上となります。充填済みのQIAGEN-tips(図「 陰イオン交換チップ」を参照)は、自然落下で作動し乾燥することがなく、プラスミド調製に要する直接の作業時間を短縮できます。QIAGENプラスミド精製システムは全体として、ユーザーおよび環境への影響が最小限となるように、フェノール、クロロホルム、臭化エチジウム、CsClなどの有害な試薬を一切使用していません。
最大500 ml培養液のアルカリ溶解に続いて(フローチャート「 QIAGEN Plasmid Kit操作手順」を参照)、キットに付属のATP依存性エクソヌクレアーゼを使用した独自の統合型消化ステップにより、混入ゲノムDNAや、欠損したり損傷したりしたコンストラクトDNAが選択的に除去できるようになります。次に、サンプルを陰イオン交換チップに充填し、プラスミドDNAが、適切な低塩濃度およびpHの条件下で選択的に結合します。RNA、タンパク質、代謝物、その他の低分子量の不純物は中塩濃度の洗浄で除去します。ゲノムDNA不含の高純度プラスミドDNAが高塩濃度のバッファーに溶出します。このDNAをイソプロパノール沈殿により濃縮、脱塩し、遠心分離で回収します。
QIAGEN Large-Construct Kitを用いて精製したDNAは、以下のようなあらゆるアプリケーションに適しています。
| 特徴 | 仕様 |
|---|---|
| Plasmid type | BAC、PAC、P1、コスミドDNA |
| Applications | サブクローニング、トランスフェクション、シークエンシングなど |
| Processing | 手動(遠心分離) |
| Culture volume/starting material | 培養液量<500 ml |
| Samples per run (throughput) | ランあたり1サンプル |
| Technology | 陰イオン交換テクノロジー |
| Time per run or prep per run | 280分 |
| Yield | <150 µg |