Type-it Microsatellite PCR Kit
快速可靠的微卫星位点多重PCR分析
快速可靠的微卫星位点多重PCR分析
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 206243
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Type-it Microsatellite PCR Kit基于具有高特异性的HotStarTaq Plus DNA Polymerase和获得专利的缓冲液系统,从而确保多重PCR的微卫星分析结果可靠且无需优化。预混液中所有成分和独特的配方确保所有位点同步的高特异性扩增。优化的操作流程使后续分析可应用高分辨率毛细管测序仪或其他电泳仪器。
Type-it Microsatellite PCR Kit使用微卫星、STR或VNTR标记对人类、动物和细菌进行快速、可靠的基因分型分析,无需冗长的优化步骤。Type-it Microsatellite PCR Kit基于专有化的QIAGEN多重技术,包括高度特异性的HotStarTaq Plus DNA Polymerase(一种化学修饰的热启动酶)和特别研发的包括Factor MP的多重缓冲液体系。这些组分确保高产量,并对所有目的微卫星位点进行可靠的多重扩增。该试剂盒包括专门用于微卫星或STRs扩增的操作流程,也包括对模板量、循环数、PCR条件和不同下游分析平台仪器细节的建议,分析平台包括琼脂糖凝胶、毛细管测序仪、Agilent Bioanalyze和QIAxcel Advanced System。
独特的Type-it Microsatellite PCR Buffer便于多重PCR产物的扩增。与常规PCR试剂不同,Type-it Microsatellite PCR Buffer含有特别研发的、比例平衡的盐和添加剂,确保反应中所有引物的退火和延伸具有同等的效率。KCl和(NH4)2SO4独特比例结合,使PCR缓冲液与常规PCR缓冲液相比,可在更宽范围的退火温度和Mg2+浓度下提供严格的引物退火条件。极大减少通过改变退火温度或Mg2+浓度的PCR优化过程,有时甚至不需要。通常使用的多重PCR优化步骤几乎不需要。缓冲液中还含有合成的Factor MP(参见 " Stable and efficient annealing"),不论引物序列如何都可进行有效的引物退火和延伸。Factor MP可提高DNA模板处的引物浓度,稳定特定结合的引物。
Type-it Microsatellite PCR Kit提供Q-Solution。这种创新的PCR添加剂通过修饰DNA的熔解行为,便于扩增难扩增模板。使用这种独特的试剂常常能完成或改进不理想的PCR。与DMSO和其他PCR添加剂不同,Q-Solution可在多种引物-模板体系中使用特定工作浓度,而不会产生毒性作用。
Type-it Microsatellite PCR Kit中专有的、特异性的操作流程,经优化后可在高分辨毛细管测序仪上进行微卫星分析,保证在常规分析中获得可靠结果,也可用于新检测的建立。可在室温下建立反应,确保更大的便利性和易用性。
Type-it Microsatellite PCR Kit确保快速、简单的多重分析构建,以获得准确、可重复的基因分型结果。无论哪种应用,包括微卫星、STR、SSR或VNTR分析,只需加入模板和引物后,根据优化方案开始热循环程序即可。反应混合物含有微卫星特异性的多重PCR所需的所有试剂,与目前的其他方法相比,无需冗长的优化步骤即可获得准确的结果(参见" Successful multiplex PCR-based genotyping analysis")。
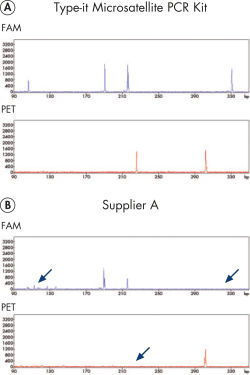
在毛细管测序仪等高分辨率检测体系上进行的微卫星分析,常常会面临产物产量不平等及荧光信号强度巨大差异等问题。Type-it Microsatellite PCR Kit突破了这些限制,保证在多重实验中对所有扩增子都有高产量。该试剂盒包括优化的操作流程,可与其他供应商的荧光引物和更好的酶溶液配合使用(参见" Reliable 13-plex STR analysis without the need for optimization")。
Type-it Microsatellite PCR Kit用于分析微卫星、STRs(短串联重复片段)、SSRs(简单序列重复)和VNTRs(数目可变串联重复序列),可用于多种研究领域,如:
| Features | Specifications |
|---|---|
| Applications | Multiplex PCR, Microsatellites, STRs, VNTRs, SSRs |
| Reaction type | PCR amplification |
| Product use | Functionally validated and developed for reliable Microsatellite Analysis |
| Mastermix | Yes |
| Real-time or endpoint | Endpoint |
| Sample/target type | Genomic DNA |
| Single or multiplex | Multiplex |
| With/without hotstart | With |