His-Strep pQE-TriSystem Vector Set
For parallel expression of His-Strep-tagged proteins in E. coli, insect, and mammalian cells
For parallel expression of His-Strep-tagged proteins in E. coli, insect, and mammalian cells
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 32942
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
The His-Strep pQE-TriSystem Vector Set allows expression of proteins with both a 6xHis tag and the Strep-tag II. The double tag allows purification by a two-step affinity purification system, enabling simple and highly efficient purification of ultrapure proteins in a standardized procedure. The system also increases throughput by eliminating the need for development and optimization of protein-specific protocols. Recombinant proteins carrying both tags are purified sequentially on Ni-NTA and Strep-Tactin matrices (see figure " Two-step affinity procedure"). The two simple affinity purifications provide fully active, full-length, and ultrapure protein that is suitable for any downstream application.
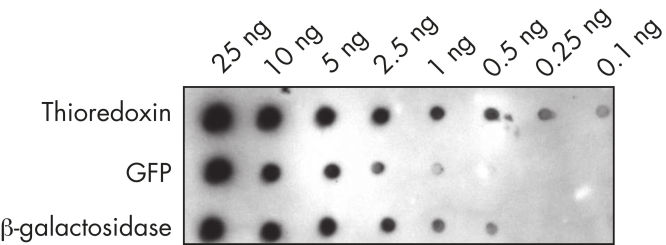
Recombinant proteins that carry two small affinity tags (the 6xHis tag and Strep-tag II) are efficiently expressed in E. coli, insect, or mammalian cells using pQE-TriSystem His-Strep vectors. After cell lysis and clearing of the lysate, proteins are initially purified using an immobilized-metal affinity chromatography (IMAC) procedure that is based on the proven 6xHis-tag-Ni-NTA interaction. After elution from the Ni-NTA matrix using imidazole, recombinant proteins (which also carry the Strep-tag II epitope) are loaded directly onto a Strep-Tactin matrix (see figure "Two-step affinity purification procedure"). No buffer exchange is required. Protein is eluted from the Strep-Tactin matrix using either biotin or desthiobiotin. Proteins can be detected with high specificity and sensitivity using mouse monoclonal Strep-tag or Anti·His antibodies (see figure " Highly sensitive detection of proteins carrying a Strep-tag").
The Two-Step Affinity Purification System is highly suited for applications where high purity is essential and is difficult to achieve with His-tag alone, for example, for proteins expressed in eucaryotic cells. The ultrahigh purity and convenience provided by the Two-Step Affinity Purification System make it the method of choice for:
| Features | Specifications |
|---|---|
| Expression species | E. coli, mammalian, cells, insect cells |
| Tag removal sequence | No |
| N- or C-terminal tag | N- and C-terminal tag |
| In-frame cloning necessary | Yes |
| Tag | 6xHis tag |
| Expression | In vivo |
| All three reading frames provided | No |