✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 31314
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Up to 300 μg His-tagged protein per column in as little as 15 minutes
- Purification under native and denaturing conditions
- Up to 95% homogeneity in one step
- Ready-to-use spin columns for rapid automated or manual processing
Product Details
Ni-NTA silica combines Ni-NTA with a macroporous silica support material optimized to suppress nonspecific hydrophobic interactions. Ni-NTA Spin Columns (His-protein purification spin columns) in the Ni-NTA Spin Kit and available separately provide Ni-NTA silica in a convenient microspin format for easy preparation of multiple samples in parallel. They provide a simple method for functional screening of engineered proteins, selection of clones expressing full-length translation products and comparison of expression levels. Each spin column can purify up to 300 µg of His-tagged protein. Like all Ni-NTA matrices, Ni-NTA spin columns can be used for one-step protein purification under native or denaturing conditions. The Ni-NTA Spin Kit is a complete kit for spin purification of His-tagged proteins. It can be automated on the QIAcube Connect (see image " QIAcube Connect").
See figures
Performance
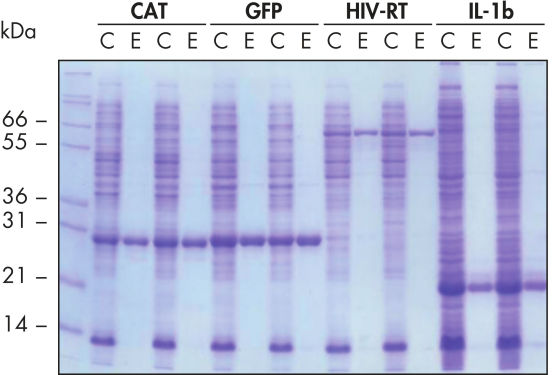
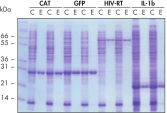
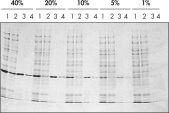
Ni-NTA Spin Columns (His-protein purification spin columns), also included in the Ni-NTA Spin Kit, allow reproducible fast automated purification (see figure “Reproducible automated purification”) at different expression levels (see figure “Purification at different expression levels”).
See figures
Principle
The QIAexpress Ni-NTA Protein Purification System, including the Ni-NTA Spin Columns and Ni-NTA Spin Kit, is based on the remarkable selectivity of patented Ni-NTA (nickel-nitrilotriacetic acid) resin for proteins containing an affinity tag of six or more histidine residues – the His tag. This technology allows one-step purification of almost any His-tagged protein from any expression system under native or denaturing conditions. NTA, which has four chelation sites for nickel ions, binds nickel more tightly than metal-chelating purification systems that only have three sites available for interaction with metal ions. The extra chelation site prevents nickel-ion leaching and results in a greater binding capacity and protein preparations with higher purity than those obtained using other metal-chelating purification systems. The QIAexpress system can be used to purify His-tagged proteins from any expression system including baculovirus, mammalian cells, yeast and bacteria.
Procedure
The purification of His-tagged proteins consists of 4 steps: cell lysis, binding, washing and elution (see figure “Ni-NTA Spin Column purification with the Ni-NTA protein purification system”). Purification of recombinant proteins using the QIAexpress system does not depend on the 3-dimensional structure of the protein or His tag. This allows one-step protein purification under either native or denaturing conditions, from dilute solutions and crude lysates. Up to 600 μl of a cell lysate are loaded onto a Ni-NTA spin column. A quick 2-minute spin binds the tagged protein to Ni-NTA silica, while most of the untagged proteins flow through. After a wash step, purified protein is eluted under mild conditions (such as pH reduction to 5.9, or addition of 100-500 mM imidazole) in a volume of 100-300 μl. Removal of the His tag is usually unnecessary since it is small and rarely immunogenic. The purified protein is ready for immediate use. Proteins can be purified from multiple small-scale expression cultures in around 30 minutes (manual procedure) or approximately 60 minutes (automated QIAcube Connect procedure). Strong denaturants and detergents can be used for efficient solubilization and purification of receptors, membrane proteins and proteins that form inclusion bodies. Reagents that allow efficient removal of nonspecifically binding contaminants can be included in wash buffers (see table). Purified proteins are eluted under mild conditions by adding 100-250 mM imidazole as competitor or by a reduction in pH.
Reagents compatible with the Ni-NTA–His interaction:
- 6 M guanidine HCl
- 8 M urea
- 2% Triton X-100
- 2% Tween 20
- 1% CHAPS
- 20 mM β-ME
- 10 mM DTT
- 50% glycerol
- 20% ethanol
- 2 M NaCl
- 4 M MgCl2
- 5 mM CaCl2
- ≤20 mM imidazole
- 20 mM TCEP
See figures
Applications
The QIAexpress Ni-NTA Protein Purification System, including Ni-NTA Spin Columns and the Ni-NTA Spin Kit, provides reliable, one-step purification of proteins suitable for any application, including:
- Structural and functional investigations
- Crystallization for determination of three-dimensional structure
- Assays involving protein–protein and protein–DNA interaction
- Immunization to produce antibodies
| Features | Ni-NTA Spin Columns | Ni-NTA Spin Kit |
| Applications | Proteomics | Proteomics |
| Bead size | 16–24 µm | 16–24 µm |
| Binding capacity | Up to 300 µg per spin column | Up to 300 µg per spin column |
| Gravity flow or spin column | Spin column | Spin column |
| Processing | Automated/manual | Automated |
| Scale | Small scale | Small scale |
| Special feature | Low-throughput screening | Up to 95% homogeneity in one step |
| Starting material | Cell lysate | Cell lysate |
| Support/matrix | Macroporus silica | Macroporus silica |
| Tag | 6xHis tag | 6xHis tag |
Supporting data and figures
Reproducible automated purification.
The indicated proteins were purified in duplicate under native conditions using Ni-NTA Spin Columns from cleared E. coli cell lysates derived from 5 ml LB cultures either manually or in an automated procedure on the QIAcube. CAT: chloramphenicol acetyl transferase; GFP: Green fluorescent protein; HIV-RT: Human immunodeficiency virus reverse transcriptase; IL-1b: Interleukin-1 beta. M: markers; C: cleared lysate (2 μl loaded per lane); E: elution fraction (3 μl loaded per lane).