✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cell and Gene Therapy Lentivirus Lysis Kit (100 rxn)
Cat no. / ID. 250323
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- One kit and one protocol solution for AAV or LV lysis enables standardization and quality control (QC) of current workflows
- Consistent and reproducible measurement of viral titers for multiple serotypes (e.g., AAV serotypes) or of different purities (e.g., from in-process supernatant to highly purified samples)
- Complete workflow combined with QIAcuity Cell and Gene Therapy (CGT) dPCR Assays and accurate measurement of AAV or LV titers on the QIAcuity Digital PCR System
Product Details
Adeno-associated virus and lentivirus are widely used viral vectors in cell and gene therapy applications. However, the process of generation and purification of the viral vectors requires precise quality control to enable safe and reliable dosing during clinical studies or patient care. The ability to accurately quantify vector titers as well as determine contaminations is key to safe and effective AAV-based and LV-based therapies.
With the introduction of direct vector lysis solutions for AAV or LV that include reagents for either 100 or 1000 reactions, we now have an optimized and standardized workflow for direct viral vector lysis through to an accurate and precise way of determining the viral titer.
Performance
We offer a complete workflow from AAV or LV particle lysis to quantification of the viral titer. The CGT Viral Vector Lysis Kit and the CGT Lentivirus Lysis Kit, with their unique formulas, give a consistent, robust, and accurate determination of the vector titer while saving hands-on time in comparison to the current workflow with a separate sample preparation step. A standardized sample processing step is key to reliable genome titration.
It works in conjunction with the QIAcuity Cell and Gene Therapy dPCR Assays, QIAcuity Digital PCR System, QIAcuity Probe PCR Kit (cat. nos. 250272, 250273), QIAcuity OneStep Advanced Probe Kit (cat. nos. 250323, 250324), and QIAcuity Nanoplate 8.5K, offering an end-to-end dPCR workflow comparable to qPCR but delivering absolute quantification of vector genome copies in your sample.
Principle
The all-in-one solution for viral vector lysis provides:
- Standardization of vector lysis with much easier standard operating procedure (SOP) implementation and QC
- Consistent quantification of titers independent of sample purities or serotypes
- Broad range of detection down to 2.5 copies/µL in QIAcuity Nanoplate 8.5k
The CGT viral vector lysis kits, in conjunction with the QIAcuity Cell and Gene Therapy dPCR Assays and dedicated master mixes running either singleplex or multiplex reactions on the QIAcuity instruments, now deliver a complete viral titer workflow.
The principle of the dPCR reaction in nanoplates is described here.
Procedure
The CGT Viral Vector Lysis Kit (cat. nos. 250272, 250273) is provided with reagents for 100 or 1000 reactions in two boxes for AAV vectors. The kit is suitable for the lysis of AAV2, AAV5, AAV6, AAV8 and AAV9. The lysates are optimized for QIAcuity Cell and Gene Therapy dPCR Assays in combination with the QIAcuity Probe PCR Kit (cat. nos. 250272, 250273).
The CGT Lentivirus Lysis Kit (cat. nos. 250323, 250324) is provided with reagents for 100 or 1000 reactions in one box for LV vectors. The lysates are optimized for QIAcuity Cell and Gene Therapy dPCR Assays in combination with the QIAcuity OneStep Advanced Probe Kit (cat. nos. 250323, 250324).
These assays enable singleplex as well as multiplex cell and gene therapy applications. The kit does not include restriction enzymes (e.g., Hpall) stated in the protocol (included in the Resources section below).
Applications
The CGT Viral Vector Lysis Kit (cat. nos. 250272, 250273) is used for the lysis of AAV and adenoviruses. The CGT Lentivirus Lysis Kit (cat. nos. 250323, 250324) is used for the lysis of LV. These kits are optimized for a range of applications, including viral vector genome titer.
Supporting data and figures
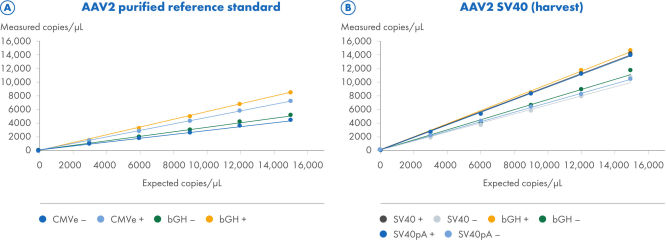
ITR digestion improves the quantification of ITR and non-ITR targets
Two AAV2 samples were processed using the CGT Viral Vector Lysis Kit and quantified using the QIAcuity Digital PCR instrument with 8.5k Nanoplates and the CGT dPCR Assays. The CGT dPCR Assays were run in triplex reactions in the FAM, HEX and Cy5 channels. The samples were serially diluted in 6 steps from 15,000 copies/µL down to 2.5 copies/µL with an R2=1.0 on 8.5k Nanoplates (A, B). Each dilution was measured in technical triplicates. Quantifications were performed with (+) and without (–) restriction digest of the ITR regions. (A) For the titration of a purified AAV2 reference standard sample, the CGT dPCR assays targeting the CMV enhancer bGH polyA regions were used. The expected copies are based on an ITR estimate determined by qPCR measurements from the reference standard supplier and not directly comparable to dPCR measurements. (B) For the titration of the AAV2-SV40 harvest sample, the CGT dPCR Assays targeting the SV40 promoter, SV40 polyA and bGH polyA regions were used.