Cat. No. / ID: 34362
特点
- 经过优化,通过 TAGZyme 酶去除表达的 His 标签
- 高效去除 His 标签:在 37°C 下只需 30 分钟即可去除 >95%
- N-末端 His 标签蛋白的高水平表达
- 高纯度终产物
- 通过 Ni-NTA 法完全去除污染物
产品详情
TAGZyme Enzyme DAPase 包含足够从多达 10 mg His 标签蛋白中高度特异性和高效去除 His 标签的酶。TAGZyme 系统可用于从使用 TAGZyme pQE-2 载体表达的含有内在 DAPase 停止点的蛋白质中去除 His 标签。
绩效
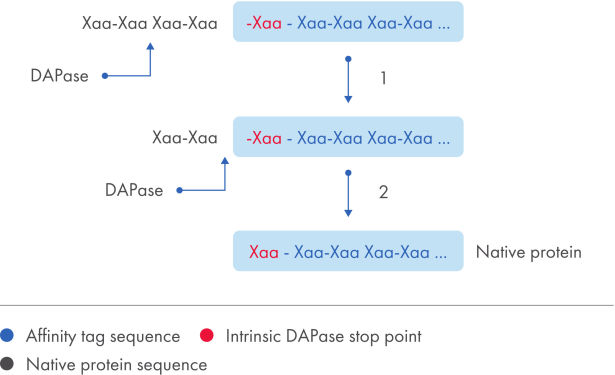
TAGZyme DAPase Enzyme 可高效地从 N-末端 His 标签起顺序去除二肽,直到使用 TAGZyme pQE-2 载体表达的“停止点”。
原理
His 标签重组蛋白已成为研究蛋白质结构和功能的宝贵工具。His 标签的小尺寸和低免疫原性意味着通常不需要去除它。然而,对于某些应用而言,例如通过 X 线或 NMR 进行的结构测定研究或治疗蛋白的生产,最好使用不含载体衍生氨基酸的蛋白产物。
TAGZyme pQE-2 载体适用于含有内在 DAPase 终止位点的蛋白质。
TAGZyme 系统能够以高度特异性和高效率从重组蛋白中去除 N-末端 His 标签。DAPase 酶用于从经过纯化的 His 标签蛋白的 N-末端顺序剪切二肽(参见图片 “His 标签去除”)。这种酶切在酶到达“停止点”时停止,后者是一种不能作为底物的氨基酸基序(参见表格“DAPase 停止点”)。
DAPase 终止位点
| 氨基酸 | DAPase 停止点 (↓) 序列* |
|---|---|
| 赖氨酸 (Lys, K) | Xaa-Xaa...Xaa-Xaa ↓ Lys-Xaa... |
| 精氨酸 (Arg, R) | Xaa-Xaa...Xaa-Xaa ↓ Arg-Xaa... |
| 脯氨酸 (Pro, P) | Xaa-Xaa...Xaa-Xaa ↓ Xaa-Xaa-Pro-Xaa... |
| 脯氨酸 (Pro, P) | Xaa-Xaa...Xaa-Xaa ↓ Xaa-Pro-Xaa-Xaa... |
| 谷氨酰胺 (Gln, Q)† | Xaa-Xaa...Xaa-Xaa ↓ Gln-Xaa... |
| 异亮氨酸 (Ile, I) | Xaa-Xaa...Xaa-Xaa ↓ Xaa-Ile-Xaa-Xaa... |
查看图表
程序
对于含有内在停止点的重组蛋白,使用 TAGZyme pQE-2 载体表达能够完全、高效地去除 N-末端 His 标签,而与 DNA 插入片段的克隆位点无关(参见图片 “His 标签去除”)。与 DAPase 酶一起孵育后,使用 Ni-NTA 基质对反应混合物进行消减固定化金属亲和层析 (immobilized-metal affinity chromatography, IMAC)(参见图片 “去标签蛋白的纯化”)。His 标签片段及 TAGZyme DAPase Enzyme(携带 C-末端 6xHis 标签)与基质结合,并且在流穿液中回收得到纯的去标签目标蛋白。
查看图表
应用
TAGZyme 系统提供了特异性剪切、重组试剂使用以及所有污染物的完全去除,使其成为生产无 His 标签蛋白的首选方法,其应用包括:
- 通过 NMR 或 X 线衍射晶体分析法进行蛋白质结构测定
- 治疗蛋白的生产
辅助数据和图表
His 标签去除。
使用 TAGZyme 酶的总体剪切策略的图示总结。DAPase 酶从含有天然停止点的蛋白质中剪切 N-末端 His 标签,以获得成熟的目标蛋白。