QIAfilter Plasmid Kits
快速纯化至多10 mg转染级质粒或柯斯质粒DNA
快速纯化至多10 mg转染级质粒或柯斯质粒DNA
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 12243
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
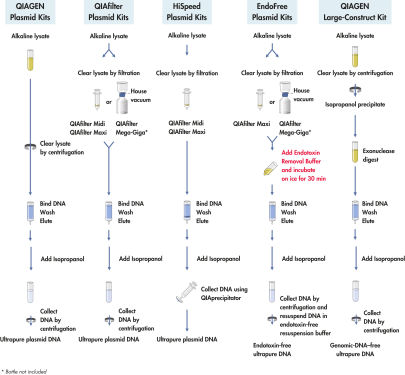
QIAfilter Plasmid Kits基于阴离子交换技术纯化质粒DNA,细菌裂解液通过过滤来澄清。该试剂盒可纯化获得至多10 mg DNA。经纯化的DNA的纯度相当于两次CsCl梯度离心方法制备的纯度,适合转染级的应用。
QIAfilter Plasmid Kit配合QIAfilter过滤条通过含有阴离子树脂的QIAGEN-tip过滤法快速澄清细菌裂解液,取代离心操作。产量可达 10 mg(Giga)、2.5 mg(Mega)、500 µg(Maxi)、或100 µg(Midi)转染级纯的高拷贝质粒DNA。(培养容量取决于质粒拷贝数、插入片段大小、宿主菌株和培养基。)
由于培养容量较大且QIAfilter Mega-Giga Cartridge自身容量有限,与QIAfilter Plasmid Giga Kits相比,QIAfilter Plasmid Mega Kits更适合纯化低拷贝质粒和柯斯质粒。
QIAfilter、HiSpeed和EndoFree Plasmid Kits提供的QIAfilter Cartridges是特别的过滤组件,用于取代离心操作澄清碱性裂解法裂解细菌细胞得到的溶液。QIAfilter Cartridges只需使用离心所需的小部分时间,即可完全去除SDS沉淀物,澄清细菌裂解液,可节省多达1小时的时间。
QIAfilter Mega-Giga Cartridges在低真空状态下工作,以最简单的方法高效澄清大体积的细菌裂解液。
QIAGEN-tips内独特的阴离子交换树脂专为纯化核酸设计。它独特的分离特性,使得到的DNA的纯度相当于连续2次CsCl梯度离心得到的DNA纯度。QIAGEN-tips利用重力流原理工作,缩短了制备质粒的手动操作时间。整套QIAGEN质粒纯化体系避免使用苯酚、氯仿、溴化乙锭和CsCl等有毒物质,减小对使用者和环境的危害。
中和的细菌裂解液直接放入QIAfilter Cartridge中,裂解液通过过滤澄清。澄清后的裂解物上样到阴离子交换柱,质粒DNA在适当的低盐条件和pH条件下与树脂选择性结合。RNA、蛋白质、代谢产物和和其它低分子量杂质用中盐洗涤液去除,用高盐缓冲液洗脱纯的质粒DNA(参见"QIAGEN Plasmid Kit procedures")。质粒DNA用异丙醇沉淀浓缩和脱盐后,离心收集。
QIAfilter Plasmid Kits纯化得到的质粒DNA高度适用于从克隆到转染等多种应用。