Type-it HRM PCR Kit
应用高分辨率熔解(HRM)分析快速、准确的检测基因突变和SNPs
应用高分辨率熔解(HRM)分析快速、准确的检测基因突变和SNPs
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 206544
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Type-it HRM PCR Kit提供可靠的解决方案,用于通过HRM分析进行快速、准确的基因分型。该试剂盒经优化,可成功分析难扩增的基因组位点。该试剂盒非常适合突变扫描,可用于筛选样本的未知突变。可简便的建立新的HRM基因分型检测。该试剂盒可稳定的获得特异性的扩增产物,减少非特异性扩增,获得可靠结果,无需进行耗时的PCR参数优化。这使得实验标准化、灵活,大大节省时间和成本。该试剂盒可配合多种具有HRM功能的real-time PCR仪使用,如Rotor-Gene Q实时荧光定量PCR分析仪、Rotor-Gene 6000、LightCycler 480和Applied Biosystems 7500 Fast System。
Type-it HRM PCR Kit经验证可准确分辨序列突变,非常适合使用新型的HRM技术明确等位基因分型。通过HRM技术,之前未知的和复杂的序列变化被认为是具有挑战性的基因分型应用,现在都可轻松分析(参见" Highly accurate genotyping")。
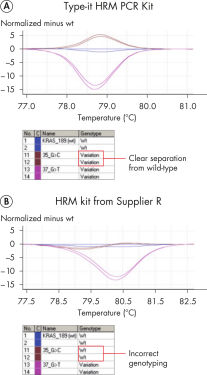
试剂盒中提供的预混液含有新型的双链DNA结合荧光染料EvaGreen,还包括经优化的HRM缓冲液、HotStarTaq Plus DNA Polymerase、Q-Solution和dNTPs。这些组分确保PCR的特异性,提供可靠的结果,甚至用于难处理的基因组位点。Type-it HRM PCR Kit优于其他供应商的试剂盒,可扩增有挑战性的细微序列突变。与其他商业化HRM预混液化合组分相比,Type-it HRM PCR Buffer的独特组分确保高度严谨和定向的引物结合(参见 " Highly specific and successful amplification of difficult genomic loci")。 与其他供应商的试剂盒不同,Type-it HRM PCR Kit可在多种real-time仪器上获得持续稳定的表现,确保对Class IV SNPs(参见" Successful genotyping of an A/T Class IV SNP")和基因突变(参见 " Successful typing of gene mutations")进行高度准确地检测。由于减少了需要重测的样本数量,可大大节省时间和成本。
Type-it HRM PCR Kit可进行高度可靠的突变扫描。未知基因突变(插入和缺失)可被轻松检测并被可靠区分,产生不同的熔解曲线(参见" Successful mutation scanning")。
HRM是一种封闭管式的、PCR后的分析方法,引起了极大地科学兴趣。HRM可根据分离(熔解)行为对双链PCR产物进行描述,随着温度升高,双链DNA(dsDNA)就会转变成单链DNA(ssDNA)。PCR产物可根据序列、长度、GC含量或链互补性,甚至是单个碱基变化进行区分。之前未知的和复杂的序列变化被认为是具有挑战性的基因分型应用,现在都可轻松分析(参见" Highly accurate genotyping")。与常规方法不同,HRM可避免PCR产物的残留污染,是一种更简单、更经济的基于探针的基因分型分析。
独特的试剂盒组成确保高度特异性的扩增(见表)。
Type-it HRM PCR Kit含有的EvaGreen是第三代饱和荧光染料,可选择性地结合到双链DNA上。与常规的SYBR® Green I相比,EvaGreen可使用更高浓度,无PCR抑制,对GC含量丰富和AT含量丰富的区域具有相同的结合能力,无明显的序列偏向性。这使得EvaGreen非常适合对多种类型的PCR产物进行HRM分析,对更低的荧光差异产生不同的熔解曲线,确保获得标准化结果。
HRM PCR Master Mix由HotStarTaq Plus DNA Polymerase和一种新型的HRM PCR缓冲液体系组成,能得到高度特异性的扩增和不同的熔解曲线,可用于难处理得基因组位点,如Class IV SNPs(参见" Successful genotyping of an A/T Class IV SNP")。确保目的PCR产物的特异性扩增;减少非特异性产物和引物二聚体的形成,如果没有去除(参见" Successful typing of gene mutations")。
新型的缓冲液(包含在预混液中)通过在每个PCR循环的退火步骤中提高特异性引物的结合比率来维持每个PCR循环的特异性扩增。KCl 和 (NH4)2SO4独特的平衡组合,使得该缓冲液在很宽的退火温度范围和Mg2+浓度范围内提供严格的引物退火条件。无需通过改变退火温度和Mg2+浓度来优化PCR反应。
Type-it HRM PCR Kit是一种简单检测难处理突变位点的有力体系(参见" Highly specific and successful amplification of difficult genomic loci")。位于GC含量高或高级二级结构区域的突变或SNPs都难以扩增,用于常规的HRM PCR方法。2x HRM PCR Master Mix包含新型的PCR添加剂Q-Solution,使用特定浓度的Q-Solution可帮助克服这些困难。Q-Solution通过修饰DNA的熔解行为,可成功扩增难处理的目标序列,无需优化。
| 组成 | 优点 |
|---|---|
| 2x HRM PCR Master Mix | 建立新的检测无需优化 专门用于突变和SNPs的HRM分析 专门用于适合HRM分析的所有循环仪 方便的反应体系构建,减少移液误差 |
| HotStarTaq Plus DNA Polymerase | 高度特异性扩增 室温下快速、简单的构建反应体系 |
| Type-it HRM PCR Buffer | 不同的熔解曲线 提高扩增特异性 |
| Q-Solution | 提高对难处理模板的扩增能力 |
| EvaGreen Dye | 新型的饱和dsDNA结合染料 不同的熔解曲线分析 |
Type-it HRM PCR Kit包括专门的、应用特异性的操作流程,预优化后用于多种real-time循环仪,如Rotor-Gene Q实时荧光定量PCR分析仪、Rotor-Gene 6000和LightCycler 480。此外,采用试剂盒提供的优化操作流程还可使用Applied Biosystems 7500 Fast System和Applied Biosystems 7900。我们的网站上提供详细的操作流程。
Type-it HRM PCR Buffer的独有特色和试剂盒提供的优化操作流程,确保一次获得成功的HRM基因分型结果。无需冗长的反应参数优化,新的HRM基因分型检测可快速、简单地标准化,并用于常规研究(参见" Successful HRM analysis without the need for optimization")。此外,Type-it HRM PCR Kit的快速循环操作流程通过缩短PCR的运行时间提高通量,可更快获得准确的结果。
该试剂盒采用即用型、预优化预混液的规格,更加方便。使用预混液可节约时间,简化反应体系构建的手工操作,通过排除移液误差和污染的可能来源提高可重复性,尽量减少移液步骤,无需繁琐的计算。使用预混液可进行室温下的反应体系构建,快速、简单。预混液中含有的HotStarTaq Plus DNA Polymerase在95°C,5-minute被激活,可在热循环程序中简单设置。
Type-it HRM PCR Kit可用于多种应用,包括:
Type-it HRM PCR Kit是多种研究领域的通用工具,包括:
| Features | Specifications |
|---|---|
| Applications | Detection of SNPs, mutations and mutation scanning |
| Product use | Functionally validated and developed for reliable detection of genetic differences using HRM |
| Reaction type | PCR amplification |
| Enzyme activity | 5'-> 3' Exonuclease activity |
| With or without ROX | Without ROX |
| Sequence specific Probe | Not necessary, EvaGreen dye for detection included in the Mastermix |
| Sample/target type | Genomic DNA |
| With/without hotstart | With |
| Mastermix | Yes |
| Real-time or endpoint | Both |