QuantiTect Whole Transcriptome Kit
对珍贵的RNA样本进行无限次real-time PCR分析
对珍贵的RNA样本进行无限次real-time PCR分析
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 207045
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QuantiTect Whole Transcriptome Kit对有限的RNA样品进行预扩增和逆转录,获得高产量cDNA,用于无限次real-time PCR基因表达分析。该试剂盒包含全转录扩增所需的全部酶和缓冲液。对Phi29聚合酶创新的修饰确保从低至1 ng RNA中制备多至40 μg cDNA。该聚合酶独特的加工过程可制备高度统一的cDNA,确保通过real-time PCR进行可靠的基因表达分析。
QuantiTect Whole Transcriptome Kit确保所有转录子高度一致的扩增,这对于基因表达分析是必需的。所有mRNA转录子以相同的形式同时在5'和3'末端扩增(参见" Equal amplification of 5' and 3' regions")。图" Preservation of transcript profile", 显示高度稳定的转录子,该图将分别应用QuantiTect Whole Transcriptome Kit全基因组扩增的cDNA和用逆转录酶制备的未扩增cDNA进行了对比。
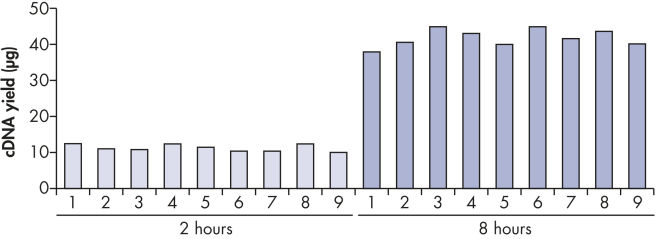
可获得多至40 μg的可重复、高产量的cDNA(参见" Reproducible cDNA yields")。能够进行无限次real-time PCR分析,并具有高度重复性的CT值(参见" Reliable real-time PCR analysis")。此外,由于cDNA比RNA更稳定,可以存档用于以后的研究工作。
| 起始材料 | 扩增时间 | 扩增产物的常规产量 | 可进行的real-time PCR次数* |
|---|---|---|---|
| 10 ng RNA | 2小时 | 至多10 µg cDNA | 1000 |
| 10 ng RNA | 8小时 | 至多40 µg cDNA | 4000 |
获得的生物样本量太少可能限制基因表达谱分析。使用QuantiTect Whole Transcriptome Kit可对少量珍贵的样品进行无限次real-time PCR分析。QuantiTect Whole Transcriptome Kit经优化,用于1 ng RNA或相当于约50个细胞RNA的全转录扩增,甚至可以用更少量的RNA,这些取决于RNA的品质和转录子的丰度。
QuantiTect Whole Transcriptome Kit含有逆转录酶、DNA聚合酶和优化的缓冲液及试剂,可对一个RNA样本的所有转录本进行扩增。此试剂盒整合了经质量验证的REPLI-g技术,通过非偏向序列扩增来合成cDNA。REPLI-g扩增采用Multiple Displacement Amplification(MDA)技术,应用独特加工的DNA聚合酶实现等温扩增(参见" Whole transcriptome amplification")。该技术确保包括5’末端在内的所有转录区的同等扩增,从而获得可用于real-time PCR的足够多的cDNA。
QuantiTect Whole Transcriptome Kit制备的cDNA可用于QuantiFast或Rotor-Gene Kits进行的real-time PCR分析,且可以储存用于以后的分析,但不适用于微阵列分析。QIAGEN提供REPLI-g Kits and Service,用于小量或珍贵样品的全基因组扩增。扩增高度一致,具有最小的序列偏好,可制备适合于基因分型和Comparative Genome Hybridization等应用的DNA。
| Features | Specifications |
|---|---|
| Amplification | Whole total RNA |
| Starting material | Purified total RNA |
| Reaction time | 5 hours or 11 hours |
| Applications | Real-time PCR, gene expression analysis |
| Minimal pipetting volume needed | 1 µl |
| Samples per run (throughput) | Mid |
| Technology | Reverse transcription followed by ligation and multiple displacement amplification (MDA) |
| Yield | 10 µg or 40 µg cDNA |
| Maximum input volume | 5 µl total RNA |
| Starting amount of total RNA | >10 ng purified total RNA |
| Reaction volume | 50 µl |