✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
dPCR CGT Assay GFP (FAM)
Cat. No. / ID: 250236
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- A broad offering of ten different wet-lab validated dPCR CGT assays
- Assays with different fluorophore choices allow multiplexing to sustain more information from one sample
- An easy and fast workflow comparable to qPCR
Product Details
Adeno-associated virus (AAV) is a widely used viral vector in gene therapy applications. However, the generation and purification of the viral vectors require rigorous quality control to enable safe and reliable dosing during clinical studies or patient care. The ability to accurately quantify vector titers and detect contamination is critical for safe and effective AAV-based gene therapies.
Performance
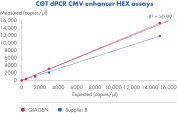
The QIAcuity Cell and Gene Therapy dPCR Assays are a broad offering of ten different wet-lab validated dPCR CGT assays that come in multiple fluorophores, enabling superior accuracy, reproducibility and a dynamic range of at least 4 orders of magnitude with speed in measuring viral titers in a multiplex setup. The assays work in conjunction with the QIAcuity Digital PCR System, QIAcuity Probe PCR Kit and QIAcuity Nanoplates, offering an end-to-end dPCR workflow comparable to qPCR, but delivering an absolute quantification of AAV vector genome copies in your sample. The assays have been designed with the requirements of biopharma manufacturing and QC in mind.
Principle
The principle of the dPCR reaction in the nanoplates is described here.
Dedicated CGT assays enable AAV quantification on the QIAcuity. These assays are validated and can be used in singleplex and multiplex reactions, and can be additionally combined with gene-of-interest assays, delivering:
- Accurate quantification down to 0.3 copies/µl
- High precision over a broad dynamic range
- High accuracy across assays (independent of fluorophores) and operators
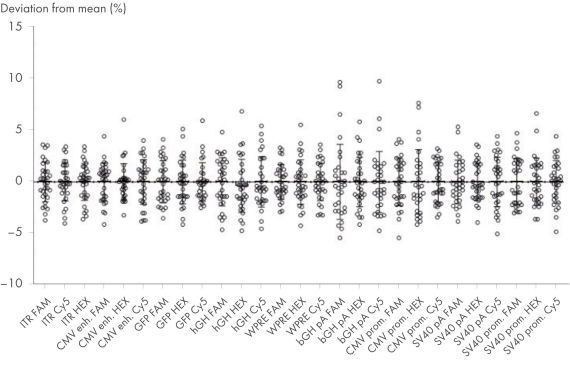
- High precision (<10% deviation from the mean) independent of fluorophores and operators
- Compatibility with both dPCR and qPCR readout
Procedure
The QIAcuity Cell and Gene Therapy dPCR Assays are provided in a 20x ready-to-use primer-probe mix, available in multiple fluorophore choices, and optimized for use with the QIAcuity Probe PCR Kit. These assays enable both singleplex and multiplex CGT applications, including absolute quantification of vector titer and assay robustness.
Applications
The QIAcuity Cell and Gene Therapy (CGT) dPCR Assays are used for various applications, including viral vector titer measurements, measuring vector genome integrity and determining potential plasmid contamination if using Amp plasmids for the production of AAVs. Incorporating these dPCR CGT Assays into the quality control process of gene therapy development means greater certainty in producing safe and potent treatments.
Supporting data and figures
High inter-assay precision
The CGT dPCR Assays were run using AAV DNA. 2500 copies/µl was used as input in a Nanoplate 8.5k. Deviations from the mean of measured copies of at least 27 replicates per assay are shown. Experiments were performed by two operators, each with at least 13 replicates.