Ni-NTA Fast Start Kit
E. coli 용해물에 있는 재조합 His 태그 단백질의 정제 및 검출에 사용
E. coli 용해물에 있는 재조합 His 태그 단백질의 정제 및 검출에 사용
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
Cat. No. / ID: 30600
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
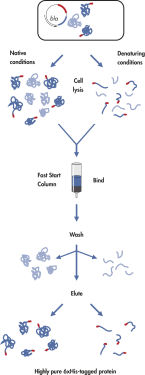
Ni-NTA Fast Start Kit는 6개 이상의 히스티딘 잔류물(연속 또는 교대)의 친화성 태그(His 태그)를 포함하는 단백질에 대한 특허받은 니켈-니트릴로트리아세트산(Nickel-Nitrilotriacetic Acid, Ni-NTA) 수지의 뛰어난 선택성을 기반으로 합니다. 이 기술을 사용하면 기본 또는 변성 조건에서 거의 모든 His 태그 단백질을 한 번에 정제할 수 있습니다. 니켈 이온에 대한 킬레이트화 부위가 4개인 NTA는 금속 이온과 상호 작용할 수 있는 부위가 3개뿐인 금속 킬레이트 정제 시스템보다 니켈을 더 단단히 결합합니다. 여분의 킬레이트화 부위는 니켈 이온 침출을 방지하여 다른 금속 킬레이트화 정제 시스템을 사용하여 얻은 것보다 순도가 더 높은 단백질 제제를 생성합니다. His 태그는 키트와 함께 제공되는 Penta·His Antibody에서 인식하는 epitope도 형성합니다.
Ni-NTA Fast Start Kit는 6xHis 태그 단백질을 신뢰할 수 있는 단일 단계로 정제하여 다음을 포함한 모든 응용 분야에 적합합니다.
| Features | Specifications |
|---|---|
| Applications | 단백질체학 |
| Support/matrix | Ni-NTA 기질 |
| Processing | 수동 |
| Binding capacity | 5~20mg/ml |
| Special feature | Ni-NTA 기술 |
| Gravity flow or spin column | 중력류 |
| Start material | 세포 용해물 |
| Tag | 6xHis tag |
| Yield | 컬럼당 단백질 10mg 미만 |