✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
Cat. No. / ID: 32903
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
특징
- C 말단 6xHis 태그가 있으면 전장 단백질만 정제됩니다.
- DHFR 융합체로서 저조하게 발현된 단백질의 발현을 위한 pQE-16 벡터
- DHFR 융합체로서 짧은 펩티드의 발현을 위한 pQE-16 벡터
제품 세부 정보
이 세트는 C 말단 6xHis 태그 단백질 발현을 위한 세 가지 벡터(pQE-16, pQE-60, pQE-70)를 제공하며, 조기 종료를 유발할 수 있는 ‘일시 중지 부위’가 있는 개방형 판독 프레임에 사용하는 것이 좋습니다. pQE-60 및 pQE-70을 사용하면 코딩 단편의 원래 시작 코돈이 pQE 벡터의 ATG를 대체하여 단백질의 실제 N 말단을 보존할 수 있습니다. 이러한 두 구축물은 PCR 또는 돌연변이 유발에 의해 삽입물의 ATG 코돈에 각각 NcoI 및 SphI 제한 부위를 주입하여 생성합니다. pQE-16을 사용하면 C 말단 6xHis 태그 DHFR 융합 단백질이 발현됩니다. 디히드로엽산 환원효소(Dihydrofolate Reductase, DHFR)은 항원성과 안정성을 강화하며, 발현이 저조한 단백질이나 단백질이 분해되기 쉬운 짧은 펩티드에 사용하는 것이 좋습니다. DHFR 자체는 마우스와 rat에서 면역원성을 거의 보이지 않기 때문에 DHFR 융합 단백질은 에피토프 스크리닝에 적합합니다.
원리
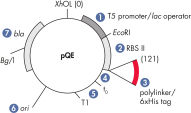
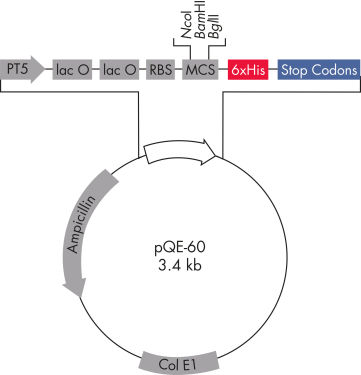
QIAexpress pQE 벡터는 강력한 파지 T5 프로모터(E. coli RNA 중합효소로 인식)와 이중 유당 작동 유전자 억제 모듈을 결합하여 E. coli에서 엄격하게 조절되는 높은 수준의 재조합 단백질 발현을 제공합니다(그림 ' QIAexpress pQE 벡터' 참조). 높은 수준의 유당 억제인자가 있는 경우 단백질 합성이 효과적으로 차단되고 세포독성 구축물의 안정성이 향상됩니다. pQE 벡터를 사용하면 재조합 단백질의 N 또는 C 말단에 6xHis 태그를 배치할 수 있습니다.
| 구성 요소 | 설명 |
| 최적화된 프로모터/작동 유전자 요소 | 파지 T5 프로모터와 유당 작동 유전자 염기서열 두 개로 구성되며, 이 염기서열은 lac 억제자 결합 가능성을 높이고 강력한 T5 프로모터를 효율적으로 억제합니다. |
| 합성 리보솜 결합 부위 RBSII | 효율적인 번역에 사용 |
| 6xHis 태그 코딩 염기서열 | 폴리링커(polylinker) 복제 영역의 5' 또는 3' |
| 번역 중지 코돈 | 발현 구축물을 편리하게 준비할 수 있도록 모든 판독 프레임에서 |
| 강력한 전사 종결자 2개 | 파지 람다의 t0 및 E. coli rrnB 오페론의 T1, 연속 판독 전사를 방지하고 발현 구축물의 안정성 보장 |
| ColE1 복제 기점 | pBR322에서 |
| Beta-lactamase gene (bla) | 암피실린 내성 부여 |
그림 참조
절차
관심 단백질을 인코딩하는 삽입물이 적절한 구조로 복제되고(자세한 정보는 QIAexpressionist 안내서 참조) 발현에 적합한 E. coli 균주로 변환됩니다. 발현은 IPTG를 첨가하면 유도됩니다. 벡터 pQE-TriSystem 구축물은 E. coli로 변환되어 곤충 세포에서 재조합 단백질 발현을 위한 셔틀 벡터로 사용되거나 포유류 세포로 변환됩니다.
응용 분야
QIAexpress Expression System은 다음을 포함하여 다양한 응용 분야에 적합한 높은 수준의 단백질 발현을
제공합니다.
- 기능 및 구조적으로 활성 상태인 단백질의 정제
- 항체 생성을 위한 변성 조건에서의 정제
- 3차원 구조 측정을 위한 결정화
- 단백질-단백질 및 단백질-DNA 상호 작용을 포함하는 분석
지원되는 데이터 및 수치
QIAeexpress pQE 벡터
Specifications
| Features | Specifications |
|---|---|
| Expression species | E. coli |
| In-frame cloning necessary | 예 |
| N- or C-terminal tag | C-terminal tag |
| Expression | In vivo |
| Special features | T5 프로모터 전사-번역 시스템 기반 |
| Tag | 6xHis tag |
| Tag removal sequence | 아니요 |
| All three reading frames provided | 예 |