EndoFree Plasmid Kits
Für die Aufreinigung von bis zu 10 mg endotoxinfreier Plasmid- oder Cosmid-DNA in hochwertiger Transfektionsqualität
Für die Aufreinigung von bis zu 10 mg endotoxinfreier Plasmid- oder Cosmid-DNA in hochwertiger Transfektionsqualität
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 12362
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
EndoFree Plasmid Kits ermöglichen die schnelle, auf Anionenaustausch basierende endotoxinfreie Aufreinigung von Plasmid-DNA. QIAfilter Cartridges ermöglichen eine schnelle Lysatbereinigung durch Filtration. Die aufgereinigte DNA übertrifft die durch zweimalige CsCl-Gradientenzentrifugation erzielte Reinheit und eignet sich für anspruchsvolle Transfektionsanwendungen. Das EndoFree Plasmid Buffer Set kann zum Ansetzen von Plasmid- oder Cosmid-DNA-Präparationen in Transfektionsqualität von 10 Mega oder 5 Giga genutzt werden.
Die EndoFree Plasmid Kits enthalten im Plasmid-Aufreinigungsverfahren einen effizienten Schritt zur Endotoxinentfernung — zur Entfernung von Lipopolysacchariden werden keine zusätzlichen Extraktionen oder Affinitätssäulen benötigt. Bakterienlysate werden durch Filtration mit einer QIAfilter Mega-Giga oder Maxi Kartusche bereinigt, und Plasmid-DNA wird mittels QIAGEN-Tips mit Anionenaustauscherharz, die nach dem Schwerkraftprinzip arbeiten, aufgereinigt. Die Ausbeute aus der Kultur beträgt bis zu 10 mg (Giga), 2,5 mg (Mega) bzw. 500 μg (Maxi) aufgereinigter DNA (das Kulturvolumen hängt von der Plasmidkopienzahl, Insertgröße, dem Wirtsstamm und dem Kulturmedium ab). Die aufgereinigte DNA ist endotoxinfrei (<0,1 EU/µg DNA).
Für die Aufreinigung von Low-Copy-Plasmiden und -Cosmiden eignet sich das EndoFree Plasmid Mega Kit besser als das EndoFree Giga Plasmid Kit, da große Kulturvolumina benötigt werden und die Kapazität der QIAfilter Mega-Giga Cartridge begrenzt ist.
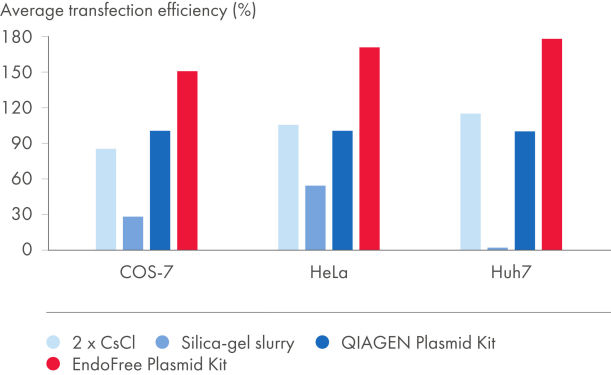
EndoFree Plasmid Kits entfernen die während der Lyse freigesetzten bakteriellen Endotoxine, welche die DNA-Transfektion in Primärzellen und sensitive Kulturzellen beeinflussen. Die aus den EndoFree Plasmid Kits gewonnene endotoxinfreie DNA eignet sich hervorragend für reproduzierbare und zuverlässige Transfektionsergebnisse (siehe Abbildungen „Zusammenhang zwischen Plasmidaufreinigungsverfahren und Transfektionseffizienz“ und „Zusammenhang zwischen Plasmidreinheit und Transfektionseffizienz“ sowie die Tabellen „Endotoxingehalt in Plasmidpräparationen“ und „EndoFree-DNA liefert hohe Transfektionseffizienz in Primärzellen“). Die hochreine endotoxinfreie DNA von QIAGEN ist auch für die Gentherapieforschung und andere anspruchsvolle Anwendungen geeignet.
| Verfahren der Plasmidpräparation | Endotoxin (EU†/µg DNA) |
Mittlere Transfektions effizienz‡ |
| EndoFree Plasmid Kit | 0,1 | 154% |
| QIAGEN Plasmid Kit | 9,3 | 100% |
| 2x CsCl | 2,6 | 99% |
| Silikagel-Aufschlämmung | 1230,0 | 24% |
| DNA-Aufreinigungsverfahren | Prozentsatz transfizierter Zellen |
| EndoFree Plasmid Kit | 21,0% ± 0,93 |
| QIAGEN Plasmid Kit | 8,1% ± 0,57 |
| Silikagel-Aufschlämmung | 5,2% ± 0,74 |
Der Grad der Endotoxin-Kontamination in aufgereinigter Plasmid-DNA hängt vom verwendeten Aufreinigungsverfahren ab (siehe Tabelle „Endotoxingehalt in Plasmidpräparationen“). Mit Silika aufgeschlämmte DNA weist extrem hohe Endotoxinwerte auf. QIAGEN, QIAfilter und HiSpeed Plasmid Kits sowie zweimalige CsCl-Ultrazentrifugation ergeben sehr reine DNA mit relativ geringen Endotoxinwerten. Um Plasmid-DNA mit einem Reinheitsgrad von <0,1 EU/µg Plasmid-DNA zu erhalten, umfassen die EndoFree Plasmid Kits einen integrierten Schritt zur Endotoxinentfernung.
Die in den QIAfilter, HiSpeed und EndoFree Plasmid Kits mitgelieferten QIAfilter Cartridges sind spezielle Filtereinheiten und ersetzen die Zentrifugation nach der alkalischen Lyse von Bakterienzellen. QIAfilter Cartridges entfernen SDS-Präzipitate vollständig und bereinigen bakterielle Lysate in einem Bruchteil der für die Zentrifugation benötigten Zeit, wodurch sich die Dauer der Plasmidaufreinigung um bis zu 1 Stunde verkürzt. QIAfilter Mega-Giga Cartridges nutzen das hausinterne Vakuum, um auch große Mengen an Bakterienlysat mit minimalem Aufwand effizient zu bereinigen (bitte beachten, dass die Flasche nicht im Lieferumfang der Kits enthalten ist). QIAfilter Maxi Cartridges haben ein Spritzenformat und die Lysate werden in Sekundenschnelle bereinigt, indem die Flüssigkeit durch den Filter gedrückt wird.
Das innovative Anionenaustauscherharz in den QIAGEN-tips wurde ausschließlich für die Aufreinigung von Nukleinsäuren entwickelt. Seine hervorragenden Trenneigenschaften führen zu einer DNA-Reinheit, die der durch zwei aufeinanderfolgende Durchgänge der CsCl-Gradientenzentrifugation erzielten Reinheit gleichwertig oder überlegen ist. Vorgepackte QIAGEN-Spitzen arbeiten nach dem Schwerkraftprinzip und trocknen nie aus, so dass der Zeitaufwand für das manuelle Ansetzen von Plasmiden minimiert wird. Im gesamten QIAGEN Plasmid-Aufreinigungssystem kommen keine toxischen Substanzen wie Phenol, Chloroform, Ethidiumbromid und CsCl zum Einsatz, was die Gefahr für den Anwender und die Umwelt minimiert.
Endotoxine, auch bekannt als Lipopolysaccharide oder LPS, sind Bestandteile der Zellmembran gramnegativer Bakterien wie E. coli (siehe Abbildung „ Bakterielle Zellwand“). Während des Lyse-Schrittes der Plasmidaufreinigung werden Endotoxine freigesetzt, die die Transfektionseffizienz bei Endotoxin-empfindlichen Zelllinien erheblich verringern (siehe Abbildungen „ Zusammenhang zwischen Plasmidaufreinigungsverfahren und Transfektionseffizienz“ und „ Zusammenhang zwischen Plasmidreinheit und Transfektionseffizienz“ sowie die Tabellen „Endotoxingehalte in Plasmidpräparationen“ und „EndoFree DNA liefert hohe Transfektionseffizienz in Primärzellen“). Außerdem können Endotoxine die Aufnahme von Plasmid-DNA in Transfektionsexperimenten beeinflussen, indem sie mit der DNA um “freies” Transfektionsreagenz konkurrieren. Endotoxine lösen auch eine unspezifische Aktivierung von Immunreaktionen in Immunzellen wie Makrophagen und B-Zellen aus, was zu einer Fehldeutung der Transfektionsergebnisse führen kann. Zu diesen Folgen gehört die induzierte Synthese von Proteinen und Lipiden wie IL-1 und Prostaglandin. Insgesamt stellen Endotoxine beim Aufbau von Transfektionsexperimenten eine nicht beeinflussbare Variable dar, die das Resultat und die Reproduzierbarkeit der Ergebnisse beeinflusst und deren Vergleich und Interpretation erschwert. In der Gentherapieforschung können Endotoxine durch Auslösung des endotoxischen Schocksyndroms und Aktivierung der Komplementkaskade Störeinflüsse verursachen.
Merkmale |
EndoFree Plasmid Maxi Kit |
EndoFree Plasmid Mega Kit |
EndoFree Plasmid Giga Kit |
| Anwendungen | Forschung zur Gentherapie, Transfektion sensitiver Zellen | Forschung zur Gentherapie, Transfektion sensitiver Zellen | Forschung zur Gentherapie, Transfektion sensitiver Zellen |
| Kulturvolumen/Ausgangsmaterial | 100–250 ml Kulturvolumen | 500 ml – 2,5 Liter Kulturvolumen | 2,5 Liter Kulturvolumen |
| Plasmidtyp | High-Copy, Low-Copy, Cosmid-DNA | High-Copy, Low-Copy, Cosmid-DNA | High-Copy, Low-Copy, Cosmid-DNA |
| Verfahren | Manuell (Schwerkraftprinzip) | Manuell (Schwerkraftprinzip) | Manuell (Schwerkraftprinzip) |
| Probe pro Lauf | 1 Probe pro Lauf | 1 Probe pro Lauf | 1 Probe pro Lauf |
| Dauer pro Lauf | 150 min | 220 min | 310 min |
| Ausbeute | <500 µg | <2,5 mg | <10 mg |
Die Bakterienzellen werden unter alkalischen Bedingungen lysiert und die Rohlysate mit der QIAfilter Cartridge bereinigt. Anschließend wird dem gefilterten Lysat der Puffer zur Endotoxinentfernung zugesetzt und das Lysat auf Eis inkubiert. Das bereinigte Lysat wird dann auf die Anionenaustauscher-Spitze aufgebracht, wo die Plasmid-DNA unter geeigneten salzarmen und pH-Bedingungen selektiv bindet. RNA, Proteine, Metaboliten und andere niedermolekulare Verunreinigungen werden durch einen Waschvorgang mit mittlerem Salzgehalt entfernt, und hochreine Plasmid-DNA wird in einem Puffer mit hohem Salzgehalt eluiert (siehe Flussdiagramm „ QIAGEN Plasmid Kit Verfahren“). Die DNA wird konzentriert, durch Isopropanol-Fällung entsalzt und durch Zentrifugation gesammelt.
Die mit den EndoFree Plasmid Kits aufgereinigte DNA eignet sich für alle anspruchsvollen Anwendungen einschließlich: