✓ Automatische Verarbeitung von Online-Bestellungen 24/7
✓ Sachkundiger und professioneller technischer und Produkt-Support
✓ Schnelle und zuverlässige (Nach-)Bestellung
Cat. No. / ID: 32903
✓ Automatische Verarbeitung von Online-Bestellungen 24/7
✓ Sachkundiger und professioneller technischer und Produkt-Support
✓ Schnelle und zuverlässige (Nach-)Bestellung
Eigenschaften
- Der C-terminale 6xHis tag gewährleistet, dass nur Volllängen-Proteine aufgereinigt werden
- pQE-16-Vektor für die Expression von schwach exprimierten Proteinen als DHFR-Fusionen
- pQE-16-Vektor für die Expression von kurzen Peptiden als DHFR-Fusionen
Angaben zum Produkt
Dieses Set enthält 3 Vektoren (pQE-16, pQE-60 und pQE-70) für die Expression von Proteinen mit C-terminalem 6xHis-tag und wird für offene Leserahmen mit „Pausenstellen“ empfohlen, die einen vorzeitigen Abbruch verursachen können. pQE-60 und pQE-70 erlauben den Austausch des ATG im pQE-Vektor gegen das originale Startcodon des codierenden Fragments, wodurch der authentische N-Terminus des Proteins erhalten bleibt. Diese beiden Konstrukte werden durch Einbringen einer NcoI- bzw. einer SphI-Restriktionsstelle am ATG-Codon des Inserts durch PCR oder Mutagenese erzeugt. pQE-16 ermöglicht die Expression von DHFR-Fusionsproteinen mit C-terminalem 6xHis-tag. DHFR (Dihydrofolatreduktase) erhöht die Antigenität und Stabilität und wird für schwach exprimierte Proteine oder kurze, proteolyseanfällige Peptide empfohlen. Da DHFR selbst eine geringe Immunogenität in Maus und Ratte zeigt, eignen sich DHFR-Fusionsproteine ideal für das Epitopscreening.
Prinzip
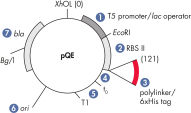
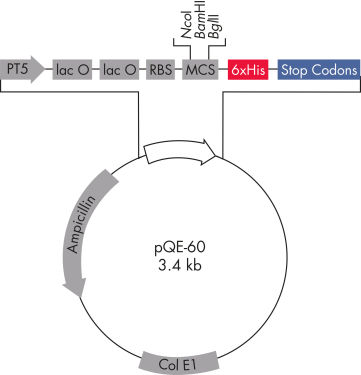
QIAexpress pQE-Vektoren kombinieren einen leistungsstarken T5-Phagen-Promotor (der durch die E. coli-RNA-Polymerase erkannt wird) mit einem doppelten lac-Operator-Repressionsmodul, um eine streng regulierte, hohe Expression rekombinanter Proteine in E. coli zu erreichen (siehe Abbildung „ QIAexpress pQE-Vektor“). Bei Vorliegen hoher Konzentrationen des lac-Repressors wird die Proteinsynthese wirksam blockiert und die Stabilität von zytotoxischen Konstrukten erhöht. Die pQE-Vektoren ermöglichen die Einführung des 6xHis-tags entweder am N- oder am C-Terminus des rekombinanten Proteins.
| Element | Beschreibung |
| Optimiertes Promotor-/Operator-Element | Besteht aus dem T5-Phagen-Promotor und zwei lac-Operator-Sequenzen, welche die Wahrscheinlichkeit der Bindung des lac-Repressors erhöhen und eine effiziente Repression des leistungsstarken T5-Promotors gewährleisten |
| Synthetische Ribosomenbindestelle RBSII | Für effiziente Translation |
| 6xHis-tag codierende Sequenz | Entweder 5' oder 3' von der Polylinker-Klonierungsregion |
| Translations-Stoppcodons | In allen Leserastern für die einfache Herstellung von Expressionskonstrukten |
| Zwei starke Transkriptionsterminatoren | t0 aus dem Lambda-Phagen und T1 aus dem rrnB-Operon von E. coli, zur Vermeidung einer Read-through-Transkription und Gewährleistung der Stabilität des Expressionskonstrukts |
| ColE1-Replikationsursprung | Aus pBR322 |
| Beta-Lactamase-Gen (bla) | Vermittelt Ampicillinresistenz |
Abbildungen ansehen
Verfahren
Inserts, die für Zielproteine codieren, werden in geeignete Konstrukte kloniert (detaillierte Informationen siehe Das QIAexpressionist Handbuch) und zur Expression in einen geeigneten E. coli-Stamm transformiert. Die Expression wird durch Zugabe von IPTG induziert. Vector pQE-TriSystem Konstrukte können in E. coli transformiert, als Shuttle-Vektor für die Expression rekombinanter Proteine in Insektenzellen verwendet oder in Säugerzellen transfiziert werden.
Anwendungen
Das QIAexpress Expressionssystem erlaubt eine hohe Expression von Proteinen für eine
Vielzahl von Applikationen, einschließlich:
- Aufreinigung funktioneller Proteine mit aktiver Konformation
- Aufreinigung unter denaturierenden Bedingungen für die Antikörperproduktion
- Kristallisierung zur Bestimmung der dreidimensionalen Struktur
- Assays mit Protein-Protein- und Protein-DNA-Interaktionen
Ergänzende Daten und Abbildungen
QIAeexpress pQE-Vektor.
Specifications
| Features | Specifications |
|---|---|
| Expression species | E. coli |
| In-frame cloning necessary | Ja |
| N- or C-terminal tag | C-terminaler Tag |
| Expression | In vivo |
| Special features | Basierend auf dem T5-Promotor-Transkriptions-Translationssystem |
| Tag | 6xHis-tag |
| Tag removal sequence | Nein |
| All three reading frames provided | Ja |