N-Terminus pQE Vector Set
Für hohe Expressionslevel von Proteinen mit N-terminalem His-tag
Für hohe Expressionslevel von Proteinen mit N-terminalem His-tag
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 32915
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Dieses Set enthält 5 Vektoren (pQE-9, pQE-30, pQE-31, pQE-32 und pQE-40) für die Expression von Proteinen mit N-terminalem His-tag. Die Vektoren pQE-30, pQE-31 und pQE-32 besitzen eine multiple Klonierungsstelle (MCS) in allen drei Leserahmen, während pQE-9 eine alternative, kürzere multiple Klonierungsstelle aufweist. Der Vektor pQE-40 ist für die Expression von DHFR-Fusionsproteinen konzipiert. DHFR erhöht sowohl die Stabilität als auch die Antigenität und wird für die Expression von schwach exprimierten Proteinen oder kurzen Peptiden empfohlen, die oft anfällig für Proteolyse sind. Da DHFR selbst in Maus und Ratte nur eine geringe Immunogenität aufweist, sind DHFR-Fusionsproteine ideal für das Epitop-Screening.
| Element | Beschreibung |
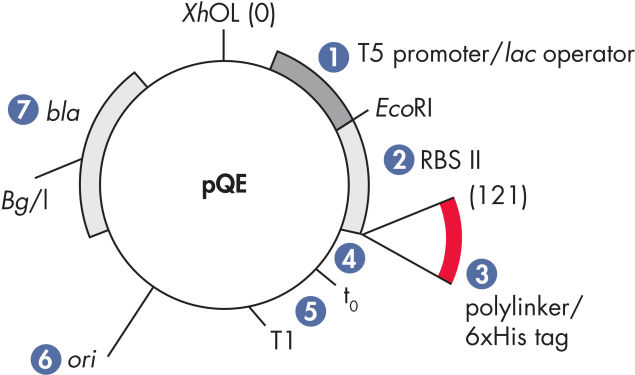
| Optimiertes Promotor-/Operatorelement | Besteht aus dem T5-Phagen-Promotor und zwei lac-Operatorsequenzen, die die Bindungswahrscheinlichkeit des lac-Repressors erhöhen und eine effiziente Unterdrückung des starken T5-Promotors gewährleisten |
| Synthetische ribosomale Bindungsstelle RBSII | Für effiziente Translation |
| 6xHis-tag-kodierende Sequenz | Entweder 5' oder 3' von der Polylinker-Klonierregion |
| Translations-Stoppcodons | In allen Leserahmen zur einfachen Herstellung von Expressionskonstrukten |
| Zwei starke Transkriptionsterminatoren | t0 vom Lambda-Phagen und T1 vom rrnB-Operon von E. coli, um eine Read-Through-Transkription zu verhindern und die Stabilität des Expressionskonstrukts zu gewährleisten |
| ColE1-Replikationsursprung | Aus pBR322 |
| Beta-Lactamase-Gen (bla) | Verleiht Ampicillin-Resistenz |
Inserts, die für die untersuchten Proteine kodieren, werden in geeignete Konstrukte kloniert (detaillierte Informationen finden Sie im QIAexpressionist-Handbuch) und zur Expression in einen geeigneten E. coli-Stamm transformiert. Die Expression wird durch Zugabe von IPTG induziert. pQE-TriSystem Vector-Konstrukte können in E. coli transformiert, als Shuttle-Vektor für die rekombinante Proteinexpression in Insektenzellen verwendet oder in Säugerzellen transfiziert werden.
Das QIAexpress Expressionssystem ermöglicht hohe Expressionslevel von Proteinen, die sich
für viele Anwendungen eignen wie:
| Features | Specifications |
|---|---|
| Expression | In vivo |
| Tag | 6xHis-tag |
| N- or C-terminal tag | N-terminaler Tag |
| Expression species | E. coli |
| Tag removal sequence | Nein |
| In-frame cloning necessary | Ja |
| All three reading frames provided | Ja |