✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 31314
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Hasta 300 μg de proteínas con etiqueta His por columna en tan solo 15 minutos
- Purificación en condiciones nativas y desnaturalizadas
- Hasta un 95 % de homogeneidad en un solo paso
- Columnas de centrifugación listas para usar para un procesamiento rápido, ya sea automatizado o manual
Product Details
La sílice Ni-NTA combina Ni-NTA con un material de soporte de sílice macroporoso optimizado para suprimir las interacciones hidrófobas inespecíficas. Las Ni-NTA Spin Columns (columnas de centrifugación para purificación de proteínas His) en el Ni-NTA Spin Kit (que se pueden comprar por separado) proporcionan sílice Ni-NTA en un cómodo formato de microcentrífuga para facilitar la preparación de varias muestras en paralelo. Proporcionan un método sencillo para el cribado funcional de proteínas manipuladas, la selección de clones que expresan productos de traducción de longitud completa y la comparación de los niveles de expresión. Cada columna de centrifugación puede purificar hasta 300 µg de proteínas con etiqueta His. Como todas las matrices Ni-NTA, las columna de centrifugación Ni-NTA se pueden usar para la purificación de proteínas en un solo paso en condiciones nativas o desnaturalizantes. El Ni-NTA Spin Kit es un kit completo para purificación mediante centrifugación de proteínas con etiqueta His. Se puede automatizar en el QIAcube Connect (véase la imagen “ QIAcube Connect”).
See figures
Performance
Las Ni-NTA Spin Columns (columna de centrifugación para purificación de proteínas His), incluidas también en el Ni-NTA Spin Kit, permiten una purificación automatizada, rápida y reproducible (consulte la figura “Purificación automatizada reproducible”) en diferentes niveles de expresión (consulte la figura “Purificación en diferentes niveles de expresión”).
See figures
Principle
El QIAexpress Ni-NTA Protein Purification System, que incluye las Ni-NTA Spin Columns y el Ni-NTA Spin Kit, se basa en la selectividad destacable de la resina patentada de Ni-NTA (níquel-ácido nitrilotriacético) para proteínas que contienen un marcador de afinidad de seis o más residuos de histidina: etiqueta His. Esta tecnología permite la purificación de un solo paso de casi cualquier proteína con etiqueta His de cualquier sistema de expresión en condiciones nativas o desnaturalizantes. El NTA, que tiene cuatro sitios de quelación para los iones de níquel, une el níquel con más fuerza que los sistemas de purificación de quelación de metales que solo tienen tres sitios disponibles para la interacción con los iones metálicos. El sitio de quelación adicional evita la lixiviación de iones de níquel y da como resultado una mayor capacidad de unión y preparaciones de proteínas con mayor pureza que las obtenidas utilizando otros sistemas de purificación de quelación de metales. El QIAexpress System se puede utilizar para purificar proteínas marcadas con His de cualquier sistema de expresión, incluidos baculovirus, células de mamíferos, levaduras y bacterias.
Procedure
La purificación de proteínas con etiqueta His consta de 4 etapas: lisis celular, unión, lavado y elución (consulte la figura “Purificación de la Ni-NTA Spin Column con el Ni-NTA Protein Purification System”). La purificación de proteínas recombinantes mediante el QIAexpress System no depende de la estructura tridimensional de la proteína ni de la etiqueta His. Esto permite la purificación de proteínas en un solo paso en condiciones nativas o desnaturalizantes, a partir de soluciones diluidas y lisadossin procesar. Se pueden cargar hasta 600 μl de un lisado celular en una columna de centrifugación Ni-NTA. Un centrifugado rápido de 2 minutos une las proteínas con etiquetas al sílice Ni-NTA, mientras que la mayoría de las proteínas no etiquetadas se filtrarán. Tras una etapa de lavado, la proteína purificada se eluye en condiciones suaves (como la reducción del pH a 5.9, o la adición de 100-500 mM de imidazol) en un volumen de 100-300 μl. La eliminación de la etiqueta His suele no ser necesaria, ya que es pequeña y en muy pocas ocasiones inmunógena. La proteína purificada está lista para un uso inmediato. Las proteínas pueden purificarse a partir de múltiples cultivos de expresión a pequeña escala en unos 30 minutos (procedimiento manual) o en unos 60 minutos (procedimiento automatizado QIAcube Connect). Se pueden utilizar desnaturalizantes y detergentes fuertes para solubilizar y purificar eficazmente receptores, proteínas de membrana y proteínas que forman cuerpos de inclusión. En los tampones de lavado pueden incluirse reactivos que permitan eliminar eficazmente los contaminantes de unión inespecíficos (consulte la tabla). Las proteínas purificadas se eluyen en condiciones suaves añadiendo 100-250 mM de imidazol como competidor o mediante una reducción del pH.
Reactivos compatibles con la interacción Ni-NTA–His:
- HCl de guanidina 6 M
- 8 M urea
- 2 % Triton X-100
- 2 % Tween 20
- 1 % CHAPS
- 20 mM β-ME
- 10 mM DTT
- Glicerol al 50 %
- Etanol al 20 %
- 2 M NaCl
- 4 M MgCl2
- 5 mM CaCl2
- ≤20 mM imidazol
- 20 mM TCEP
See figures
Applications
El QIAexpress Ni-NTA Protein Purification System, incluidas las Ni-NTA Spin Columns y el Ni-NTA Spin Kit, proporciona purificación fiable en un solo paso de proteínas aptas para cualquier aplicación, como por ejemplo:
- Investigaciones estructurales y funcionales
- Cristalización para la determinación de estructuras tridimensionales
- Ensayos que implican interacciones proteína–proteína y proteína–ADN
- Inmunización para producir anticuerpos
| Features | Ni-NTA Spin Columns | Ni-NTA Spin Kit |
| Aplicaciones | Proteómica | Proteómica |
| Tamaño de microesfera | 16–24 µm | 16–24 µm |
| Capacidad de unión | Hasta 300 µg por columna de centrifugación | Hasta 300 µg por columna de centrifugación |
| Flujo de gravedad o columna de centrifugación | Columna de centrifugación | Columna de centrifugación |
| Procesamiento | Automatizado/manual | Automatizado |
| Escala | Escala pequeña | Escala pequeña |
| Característica especial | Cribado de bajo rendimiento | Hasta 95 % de homogeneidad en un solo paso |
| Material de inicio | Lisado celular | Lisado celular |
| Soporte/matriz | Sílice macroporoso | Sílice macroporoso |
| Etiqueta | Etiqueta 6xHis | Etiqueta 6xHis |
Supporting data and figures
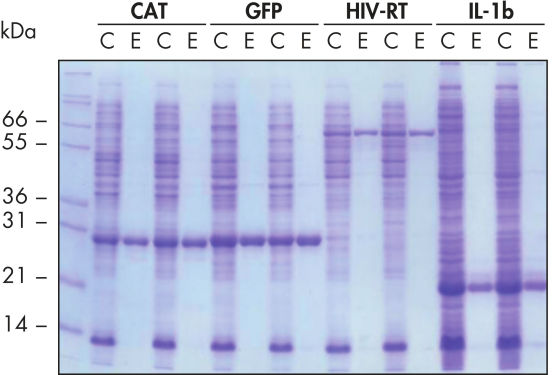
Purificación automatizada reproducible.
Las proteínas indicadas se purificaron por duplicado en condiciones nativas utilizando las Ni-NTA Spin Columns a partir de lisados celulares clarificados de E. coli derivados de cultivos LB de 5 ml, o bien manualmente o mediante un procedimiento automatizado en el QIAcube. CAT: cloranfenicol acetiltransferasa; GFP: proteína verde fluorescente; VIH-TI: transcriptasa inversa del virus de la inmunodeficiencia humana; IL-1b: interleucina-1 beta. M: marcadores; C: lisado clarificado (2 μl de carga por carril); E: fracción de elución (3 μl de carga por carril).