✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
特点
- 制备时间少于 60 分钟
- 无需离心实现裂解物澄清和异丙醇沉淀
- 沉淀过程中无 DNA 颗粒损失风险
- 高拷贝质粒 DNA 产量高达 750 µg
- LyseBlue 可实现最优裂解和最大 DNA 产量
- 无需真空歧管即可进行大规模质粒制备
产品详情
HiSpeed Plasmid Kits 无需离心即可提供基于阴离子交换的快速大规模质粒 DNA 制备。纯化后的 DNA 与通过 2 论 CsCl 梯度离心得到的 DNA 相当,适合用于转染级应用。
绩效
HiSpeed Plasmid Kits 包含 QIAfilter Cartridges、HiSpeed Tips 和 QIAprecipitator 模块,无需真空歧管即可快速进行大规模质粒制备。注射器式 QIAfilter 和 QIAprecipitator 模块取代了传统阴离子交换程序中的离心步骤,使质粒纯化更快、更方便。可从 150 ml – 250 ml 或 50 ml – 150 ml 培养液中分别纯化高达 750 µg (Maxi) 或 200 µg (Midi) 高拷贝质粒 DNA(培养液量取决于质粒拷贝数、插入片段大小、宿主菌株和培养基)。HiSpeed Tip 设计可实现很高的流速,让质粒纯化的 DNA 结合、洗涤和洗脱步骤执行速度更快。
HiSpeed Tips 中独特的阴离子交换树脂专为纯化核酸而开发。其卓越的分离性能可使 DNA 纯度相当于或优于连续两轮 CsCI 梯度离心所获的纯度。
原理
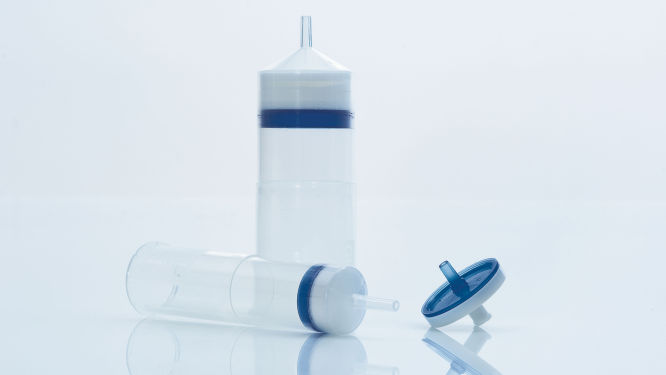
QIAfilter Cartridges(见图“ QIAfilter Cartridge”)是专用过滤装置,设计用于替代细菌细胞碱裂解后的离心步骤。QIAfilter Cartridges 只需离心分离所需的一小部分时间即可完全去除 SDS 沉淀并澄清细菌裂解物。预包装 HiSpeed Tips 靠重力流工作,永远不会干涸,从而尽量减少质粒制备所需的手动操作时间。
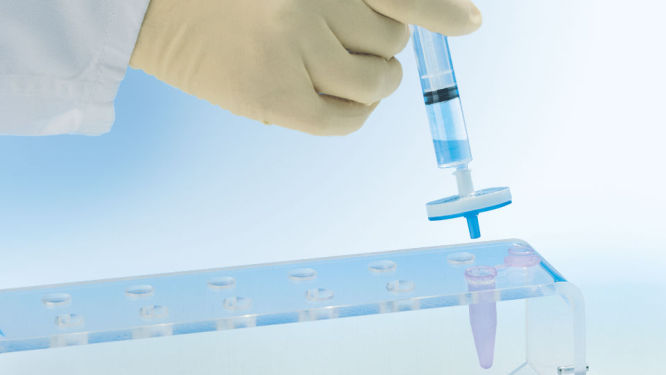
独特的 QIAprecipitator 模块(见图“ QIAprecipitator 模块”)取代了传统上纯化后用于收集异丙醇沉淀 DNA 的离心步骤。QIAprecipitator 模块在异丙醇-缓冲液混合物流过时捕获沉淀的 DNA。然后用 TE 缓冲液或水将 DNA 从 QIAprecipitator 中简单地洗脱到微型离心管中。这一独特模块还消除了颗粒损失风险,该风险可能发生在离心后倾析上清液时。
| 特点 | HiSpeed Plasmid Midi Kit | HiSpeed Plasmid Maxi Kit |
|---|---|---|
| 应用 | 转染、克隆、测序、基因沉默 | 转染、克隆、测序、基因沉默 |
| 培养体积/起始材料 | 50 µl – 150 ml 培养液量 | 150 µl – 250 ml 培养液量 |
| 质粒类型 | 高拷贝柯斯质粒 DNA | 高拷贝柯斯质粒 DNA |
| 处理 | 手动(过滤) | 手动(过滤) |
| 每次运行的样本数 | 每次运行 1 个样本 | 每次运行 1 个样本 |
| 技术 | 阴离子交换技术 | 阴离子交换技术 |
| 每次运行的时间 | 45 分钟 | 60 分钟 |
| 产量 | <200 µg | <750 µg |
程序
中和的细菌裂解物在 QIAfilter Cartridge 中孵育,数秒内通过过滤澄清。将滤液倒入 HiSpeed 吸头进行质粒 DNA 纯化(见流程图“QIAGEN Plasmid Kit 程序”)。将洗脱 DNA 与异丙醇混合,用提供的注射器注入 QIAprecipitator 模块。然后用 TE 缓冲液或水将浓缩并脱盐的 DNA 从 QIAprecipitator 中直接洗脱到一个微型离心管中。
应用
使用 HiSpeed Plasmid Kits 纯化的 DNA 在从克隆和测序到转染和质粒介导基因沉默等所有应用中都能获得优异的结果。
辅助数据和图表
QIAprecipitator 模块。