Pf/Non-Pf Detection Assay Kit
Cat no. / ID. 224113
Features
- Detects Plasmodium falciparum and non-falciparum species in a single reaction in a primary assay
- Differentiates P. vivax, P. malariae, P. ovale and P. knowlesi in a secondary assay
- Suitable for liquid whole blood and dried blood spots (DBS)
- Direct qPCR from sample to save time and reagent costs
- High sensitivity, detecting down to 1 parasite/µL
Product Details
The Pf/Non-Pf Detection Assay and Pv/Pm/Po/Pk Detection Assay together with QIAprep& Plasmodium Kit provide a fast, direct, and cost-effective RT-qPCR approach for detecting and differentiating Plasmodium species.
The Pf/Non-Pf Detection Assay screens for P. falciparum vs. non-P. falciparum species. Additionally this assay includes RNase P as an in-process control.
If non-falciparum species are detected, the Pv/Pm/Po/Pk Detection Assay differentiates between P. vivax, P. malariae, P. ovale, and P. knowlesi.
The assays are best suited for use with the QIAprep& Plasmodium Kit.
Would you like to learn more about the product from one of our specialists? Contact us, and we will get in touch with you shortly.
Performance
The Pf/Non-Pf Detection Assay delivers high sensitivity and specificity for detecting Plasmodium falciparum and non-falciparum species in a single reaction at 1 parasite/µL. Even if falciparum and non-falciparum species are present together in one sample, the assay can reliably detect them.
If the Pf/Non-Pf Detection Assay indicates presence of non-falciparum species or in cases where P. falciparum is not common in the region of interest, the Pv/Pm/Po/Pk Detection Assay would be the assay of choice for identifying non-falciparum species. This assay provides species-level identification for P. vivax, P. malariae, P. ovale, and P. knowlesi in a single reaction. It maintains high sensitivity, ensuring accurate detection at 1 parasite/µL.
Both assays are suitable for use with liquid blood or dried blood spots (DBS), making them ideal for applications such as surveillance and epidemiological research. The assays are designed for broad compatibility with most real-time qPCR platforms, allowing seamless integration into existing molecular testing workflows. With results available in approximately one to one and a half hours, these assays support rapid decision-making in malaria research and control efforts.
| Hit rates | DBS direct WF | DBS Elution WF | Whole Blood WF | Sedimentation WF | |||
| Bloodstain Card | Classic Card | Bloodstain Card | Classic Card | --- | --- | ||
| Artifical DNA Pk | 200 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% |
| 20 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| 5 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| Artifical DNA Pm | 200 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% |
| 20 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| 5 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| Artificial DNA Po | 200 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% |
| 20 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| 5 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| Artifical DNA Pv | 200 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% |
| 20 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
| 5 copies/rxn | 100% | 100% | 100% | 100% | 100% | 100% | |
The species differentiation assay was tested with artificial DNA on negative blood samples using the QIAprep& Plasmodium Kit and the different workflows. The artificial DNA was spiked into the mastermix. The DBS workflows were processed with two different card types (QIAcard Bloodstain and QIAcard FTA Classic). Pm = Plasmodium malariae, Po = Plasmodium ovale, Pv= Plasmodium vivax, Pk = Plasmodium knowlesi, WF = workflow, rxn = reaction
Principle
The Pf/Non-Pf Detection Assay and Pv/Pm/Po/Pk Detection Assay are qPCR-based multiplex assays that detect and differentiate Plasmodium species directly from liquid blood or dried blood spots (DBS). This streamlined approach reduces costs, hands-on time and minimizes sample processing steps compared to traditional PCR methods.
The Pf/Non-Pf Detection Assay, distinguishes between P. falciparum and non-falciparum species in a single reaction. The Pv/Pm/Po/Pk Detection assay identifies and distinguishes between P. vivax, P. malariae, P. ovale and P. knowlesi. The detection system relies on species-specific primers and fluorescent probes that generate signals in distinct detection channels.
Procedure
The Pf/Non-Pf Detection Assay and Pv/Pm/Po/Pk Detection Assay follow a simple and efficient qPCR workflow. Add 1 µL of the assay mix to a 20 µL reaction, along with the sample – either liquid blood or a dried blood spot (DBS) punch. Load the prepared reaction into a real-time PCR cycler and run it using the recommended cycling conditions.
The Pf/Non-Pf Detection Assay detects P. falciparum in the FAM channel and non-falciparum species in the Cy5 channel, while the internal control (HEX channel) confirms sample integrity and PCR efficiency.
If P. falciparum is not present in the geographic region of investigation or non-falciparum species have been detected with the primary assay, you can identify the particular species: P. vivax, P. malariae, P. ovale and P. knowlesi with the Pv/Pm/Po/Pk Detection Assay.
The entire process provides clear results in approximately one and a half hours, ensuring rapid, reliable species identification for malaria research and surveillance.
Supporting data and figures
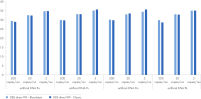
Similar sensitivity in mixed and non-mixed infections with the Pf/Non-Pf Detection Assay
Several dilutions of P. falciparum DNA and non-falciparum synthetic DNA were mixed to represent mixed infections at different parasitemias. On the right side the different P. falciparum DNA dilutions are depicted (light blue), in dark blue the non-falciparum dilutions are depicted.
Different mixtures were created (high P. falciparum parasitemia, intermediate P. falciparum parasitemia, low P. falciparum parasitemia). For non-falciparum the concentrations were: intermediate, low, very low. Next, all concentrations between P. falciparum and non-falciparum were combined. None of our simulated mixed infections showed a decrease in sensitivity in comparison to the non-mixed samples. All samples were processed with the sedimentation workflow with negative blood in the background.