Products

Cat. No. / ID: 204654

Cat. No. / ID: 204756
Features
- 同一チューブ内で再興種類のターゲットを高感度で検出
- 時間を最大50%まで短縮し迅速な結果
- 至適化実験なしに良好なマルチプレックスPCRを実現
- 発現量に差異がある複数のターゲットでも信頼性のある定量が可能
Product Details
QuantiFast Multiplex PCR Kit は、マルチプレックスリアルタイムPCRまたは 2ステップRT-PCRにより、同一チューブ中で最高4種類のcDNAまたはゲノムDNAターゲットを高速かつ確実に定量します。Q-bondテクノロジーと至適化済みのマスターミックスにより、短いRamping Time機能を持つ高速サイクラーだけではなくほとんどのサイクラーで性能を損なうことなく高速なマルチプレックスPCR を行なえます。ホットスタートと即使用可能なマスターミックス中の独自のPCRバッファーシステムの組み合わせは、至適化なしにほとんどのサイクラーで高感度なqRT-PCRを実現します。2種類のキットが入手できます:蛍光補正のためにROXが必要なサイクラー用QuantiFast Multiplex PCR Kit およびその他のサイクラー用のQuantiFast Multiplex PCR +R Kit。マスターミックスは2~8 ℃で保存でき、簡便に取り扱えます。
Performance
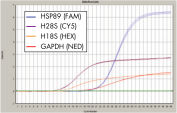
QuantiFast Multiplex PCR Kit に添付の特化されたマスターミックスにより、迅速なマルチプレックス反応のセットアップができ、singleplex PCRデータに匹敵するマルチプレックスPCRデータを提供し、初めての実験でも良好な結果が得られます(図 " triplexとsingleplex PCRで同等の結果を実現")。本キットを用いると、テンプレート量のわずかな差異を明確に判別できます。QuantiFast Multiplex PCR Kitsは、発現量が大きく異なる2種類のターゲットで、連続2倍希釈したテンプレート量でさえも正確に定量できます(図 " テンプレート量の連続2倍希釈液でも直線性の高いCT 値")。
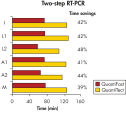
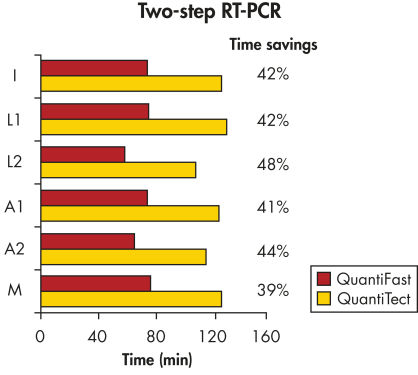
QuantiFast Multiplex PCR Kit はPCR時間を最大50%まで短縮できるため、結果がより速く得られます(図 " PCR時間を顕著に短縮")。わずか10コピーのターゲットを60分で検出できます(図 " 広いダイナミックレンジを持つ高感度なduplex PCR")。従って、サンプル数を大幅に増やしたり、他の実験者と1 台のサイクラーを効率的に共有できます。最高4 種類のターゲットのマルチプレックスリアルタイムPCRにおいて性能を損なうことなく、迅速に結果が得られます(図" 感度を損なわずに増幅")。
See figures
Principle
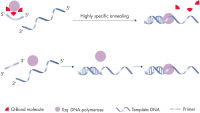
QuantiFast Multiplex PCR Kitsでは最適化実験の必要はなく、標準サイクラーまたは高速サイクラーのどちらでも幅広いダイナミックレンジの高感度かつ迅速な結果が得られます(フローチャート " QIAGEN multiplex kits")。特別に開発された高速PCR 用バッファーは斬新なQ-Bondを含有し、変性、アニーリングおよびエクステンション時間を顕著に短縮します(図" プライマーの高速なアニーリング")。
別々の反応ではなく同一反応内でコントロール遺伝子と標的遺伝子を増幅することで、マニュアルでの作業によるエラーが最小限に抑えられ、遺伝子定量の信頼性が高くなります。QuantiFast Multiplex PCR Bufferに入っている画期的な合成添加剤Factor MPとK+およびNH4+のイオン配合比により、プライマーとプローブが核酸テンプレートに効率的かつ安定してアニーリングし、高いPCR効率と感度を実現します(図 " ユニークなPCRバッファー")。さらに、HotStarTaq Plus DNA Polymeraseは厳密なホットスタートを行なえるため、非特異的な産物の形成を抑えます。
| 成分 | 特長 | 利点 |
|---|---|---|
| HotStarTaq Plus DNA Polymerase | 95℃、5分の活性化 | 室温での定量PCRのセットアップ |
| QuantiFast Multiplex PCR Buffer | NH4+イオンおよび K+イオンの配合バランス | 特異性の高いプライマーのアニーリングで信頼性の高いPCR結果 |
| 合成添加剤Factor MP | 同一チューブで4遺伝子まで信頼できるマルチプレックス解析が可能 | |
| ユニークなQ-Bond を含む | PCR反応時間が短縮されるため迅速に結果が得られ、1日あたりの反応数を増やせる | |
| ROX 色素† | Applied Biosystems製装置およびAgilent製装置での蛍光シグナルを補正 | ROXが必要なサイクラーで正確な定量。他のリアルタイムサイクラーでの反応を妨害しない |
See figures
Procedure
QuantiFast Multiplex PCR Kit は反応条件やサイクリング条件の至適化が不要で即使用可能なマスターミックスです。テンプレートRNA、プライマープローブセットをマスターミックスに加えハンドブックのプロトコール通りに操作するだけで、どのリアルタイムサイクラーでも迅速に信頼性の高い結果が得られます。マスターミックス中にROX passive reference dye が入ったキットまたは入っていないキットをお求めいただけますので、実質的にあらゆるリアルタイムサイクラーで使用できます(表参照)。ROX濃度が至適化されているため、コピー数が少ない場合の検出でも自動データ解析を行なえます。
| ROX 色素 | キット | 対応するサイクラー |
|---|---|---|
| マスターミックスに添加済み | QuantiFast Multiplex PCR Kit | Applied Biosystems 7500以外のApplied Biosystemsの全てのサイクラー |
| 別チューブで添付 | QuantiFast Multiplex PCR +R Kit | Applied Biosystems 7500 および Bio-Rad、Cepheid、Eppendorf、QIAGEN、Roche、Agilent、その他の会社のサイクラー |
2ステップリアルタイムRT-PCRで最適な結果を得るために、QuantiTect Reverse Transcription Kitを使用してcDNAを合成することをお奨めします。QuantiTect Reverse Transcription Kitは、ゲノムDNAの混入を除去しながらわずか20分で迅速にcDNA を合成します。
QuantiFast Probe Assaysは加水分解プローブ検出をベースにしたデザイン済みのゲノムワイドなアッセイです。QuantiFast Probe Assaysは、duplexの2ステップリアルタイムRT-PCRで確かな結果が得られるようQuantiFast Multiplex PCR Kit に同梱されています。
Applications
Supporting data and figures
Significantly reduced PCR times.
Specifications
| Features | Specifications |
|---|---|
| Applications | Quantification of cDNA or genomic DNA targets in a multiplex real-time PCR |
| Thermal cycler | Real-time cyclers dedicated for multiplex PCR (e.g., most Applied Biosystems real-time PCR cyclers, Roche LightCycler® 480, iCycler iQ®, Rotor-Gene) |
| Real-time or endpoint | Real-time |
| Sample/target type | cDNA, DNA |
| SYBR Green I or sequence-specific probes | Sequence-specific probes |
| Reaction type | Real-time two-step RT-PCR |
| Single or multiplex | Multiplex |
| With or without ROX | Available with ROX in master mix and with ROX as separate vial |