✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
Cat. No. / ID: 32903
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
特徴
- C末端6xHisタグにより、全長タンパク質のみが確実に精製されます
- 低発現タンパク質をDHFR融合体として発現させるためのpQE-16ベクター
- 短いペプチドをDHFR融合体として発現させるためのpQE-16ベクター
製品詳細
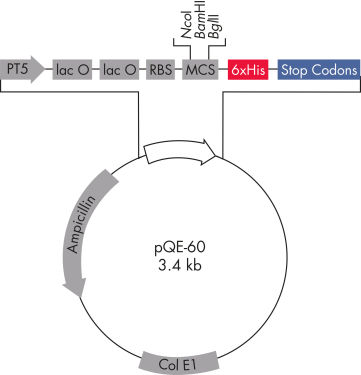
このセットには、C末端6xHisタグ付きタンパク質発現用の3つのベクター(pQE-16、pQE-60、pQE-70)が含まれており、早期終了を引き起こす可能性がある「一時停止サイト」を持つオープンリーディングフレームに推奨されます。pQE-60とpQE-70は、コード化断片の元の開始コドンをpQEベクターのATGに置き換えることができ、タンパク質の本来のN末端を保持します。これら2つのコンストラクトは、PCRまたは突然変異誘発により、インサートのATGコドンにそれぞれNcoIおよびSphI制限部位を導入することで作成されます。pQE-16は、C末端6xHisタグ付きDHFR融合タンパク質の発現を可能にします。DHFR (ジヒドロ葉酸還元酵素)は抗原性と安定性を強化するので、発現量の少ないタンパク質やタンパク質分解を受けやすい短いペプチドに推奨されます。DHFR自体はマウスやラットではほとんど免疫原性を示さないため、DHFR融合タンパク質はエピトープスクリーニングに最適です。
原理
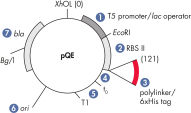
QIAexpress pQEベクターは、強力なファージT5プロモーター(E. coli RNAポリメラーゼによって認識される)とdoublelac オペレーター抑制モジュールを組み合わせ、E. coliで、組み換えタンパク質の厳密に制御された高レベル発現を実現します(図 QIAexpress pQEベクターを参照)。タンパク質合成は、高レベルlacリプレッサー存在下で効果的に阻害され、細胞毒性コンストラクトの安定性が強化されます。 pQEベクターは、組み換えタンパク質のN末端またはC末端のいずれかに6xHisタグを配置できます。
| 要素 | 説明 |
| 最適化したプロモーター/オペレーター要素 | ファージT5プロモーターと2つのlacオペレーター配列で構成され、lacリプレッサー結合確率を高め、強力なT5プロモーターの効率的な抑制を保証します |
| 合成リボゾーム結合部位RBSII | 効率的な翻訳用 |
| 6xHisタグコード化配列 | ポリリンカークローニング領域の5'または3'のいずれか |
| 翻訳停止コドン | 発現コンストラクトの調製に便利な全リーディングフレーム内 |
| 2つの強力な転写ターミネーター | λファージ由来のt0とE. coliのrrnBオペロン由来のT1は、リードスルー転写を防ぎ、発現コンストラクトの安定性を確保します |
| ColE1複製起点 | pBR322から |
| β-ラクタマーゼ遺伝子(bla) | アンピシリン耐性を付与 |
図参照
操作手順
目的のタンパク質をコードするインサートを適切なコンストラクトにクローニングし(詳細情報については、QIAexpressionistハンドブックを参照)、発現に適したE. coli株に形質転換します。発現はIPTGを添加すると誘導されます。ベクターpQE-TriSystemコンストラクトは、E. coliへ形質転換されるか、昆虫細胞で組み換えタンパク質発現用のシャトルベクターとして使用されるか、哺乳動物細胞にトランスフェクションされます。
アプリケーション
QIAexpress Expressionシステムは、以下のような
数多くのアプリケーションに適したタンパク質の高レベル発現を実現します。
- 機能的で立体構造的に活性なタンパク質の精製
- 抗体生産のための変性条件下での精製
- 三次元構造決定のための結晶化
- タンパク質-タンパク質およびタンパク質-DNA相互作用を含むアッセイ
裏付けデータと数値
QIAeexpress pQEベクター
Specifications
| Features | Specifications |
|---|---|
| Expression species | E. coli |
| In-frame cloning necessary | はい |
| N- or C-terminal tag | C末端タグ |
| Expression | In vivo |
| Special features | T5プロモーター転写翻訳システムに基づく |
| Tag | 6xHisタグ |
| Tag removal sequence | いいえ |
| All three reading frames provided | はい |