✓ Procesamiento automático sin interrupción de pedidos en línea
✓ Servicio técnico y para productos experto y profesional
✓ Realización y repetición de pedidos rápidas y fiables
HiSpeed Plasmid Midi Kit (25)
N.º de cat. / ID. 12643
✓ Procesamiento automático sin interrupción de pedidos en línea
✓ Servicio técnico y para productos experto y profesional
✓ Realización y repetición de pedidos rápidas y fiables
Características
- Menos de 60 minutos de tiempo de preparación
- Aclaramiento de lisado y precipitación de isopropanol sin centrifugación
- Sin riesgo de pérdida de sedimento de ADN durante la precipitación
- Rendimiento de ADN plasmídico de alto número de copias de hasta 750 µg
- LyseBlue para lisado óptimo y máximo rendimiento de ADN
- Preparación de plásmidos a gran escala sin colector de vacío
Detalles del producto
Los HiSpeed Plasmid Kits proporcionan una preparación de ADN plasmídico rápido, a gran escala, de intercambio aniónico sin centrifugación. El ADN purificado es equivalente al obtenido por centrifugación en gradiente 2 × CsCl y es apto para aplicaciones que requieran grado de transfección.
Rendimiento
Los HiSpeed Plasmid Kits contienen QIAfilter Cartridges, HiSpeed Tips y módulos QIAprecipitator para una preparación de plásmidos rápida y a gran escala sin el colector de vacío. Los módulos QIAfilter y QIAprecipitator con formato de jeringa sustituyen los pasos de centrifugación en el procedimiento clásico de intercambio aniónico, haciendo que la purificación de plásmidos sea más rápida y cómoda. Se pueden purificar hasta 750 µg (maxi) o 200 µg (midi) de ADN plasmídico de alto número de copias a partir de 150-250 ml o 50-150 ml de cultivo, respectivamente (los volúmenes de cultivo dependen del número de copias del plásmido, el tamaño del inserto, la cepa huésped y el medio de cultivo). El diseño de HiSpeed Tip permite una tasa de flujo muy alta, lo que permite los pasos de unión de ADN, el lavado y la elución para la purificación de plásmidos para continuar con más rapidez.
La exclusiva resina de intercambio aniónico de HiSpeed Tips se ha desarrollado exclusivamente para la purificación de ácidos nucleicos. Sus excepcionales propiedades de separación dan como resultado una pureza del ADN equivalente o superior a la obtenida mediante dos rondas sucesivas de centrifugación en gradiente de CsCl.
Principio
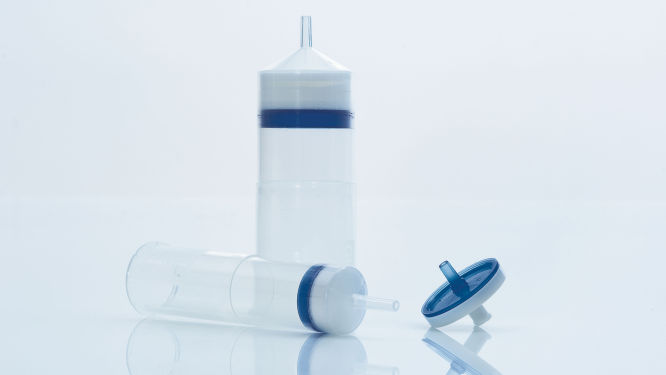
Los QIAfilter Cartridges (véase la figura “ QIAfilter Cartridge”), son unidades de filtrado especiales diseñadas para sustituir el paso de centrifugación después de la lisis alcalina de células bacterianas. Los QIAfilter Cartridges eliminan completamente los precipitados de SDS y aclaran los lisados bacterianos en una fracción del tiempo necesario para la centrifugación. Las HiSpeed Tips preenvasadas funcionan mediante flujo de gravedad y nunca se secan, lo que reduce al mínimo el tiempo de manipulación necesario para la preparación de plásmidos.
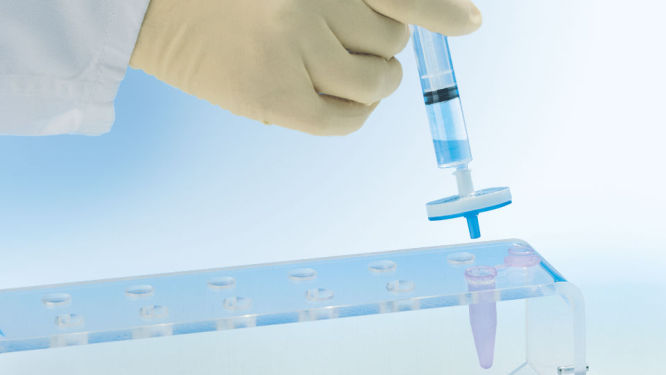
El exclusivo módulo QIAprecipitator (véase la figura “ Módulo QIAprecipitator”) sustituye al paso de centrifugación utilizado tradicionalmente para recoger el ADN precipitado con isopropanol tras la purificación. El módulo QIAprecipitator atrapa el ADN precipitado, mientras que la mezcla de isopropanol y tampón fluye a través del mismo. A continuación, el ADN se eluye simplemente del QIAprecipitador en un tubo de microcentrifugadora con tampón TE o agua. Este módulo exclusivo también elimina el riesgo de pérdida de sedimento, que puede producirse durante la decantación del sobrenadante tras la centrifugación.
| Características | HiSpeed Plasmid Midi Kit | HiSpeed Plasmid Maxi Kit |
|---|---|---|
| Aplicaciones | Transfección, clonación, secuenciación, silenciamiento génico | Transfección, clonación, secuenciación, silenciamiento génico |
| Material inicial/volumen de cultivo | 50 µl-150 ml de volumen de cultivo | 150 µl-250 ml de volumen de cultivo |
| Tipo de plásmido | ADN cósmido de número alto de copias | ADN cósmido de número alto de copias |
| Procesamiento | Manual (filtración) | Manual (filtración) |
| Muestra por serie | 1 muestra por serie | 1 muestra por serie |
| Tecnología | Tecnología de intercambio aniónico | Tecnología de intercambio aniónico |
| Tiempo por serie | 45 min | 60 min |
| Rendimiento | <200 µg | <750 µg |
Procedimiento
Los lisados bacterianos neutralizados se incuban en el QIAfilter Cartridge y se aclaran en segundos mediante filtración. El filtrado se aplica a una punta HiSpeed para la purificación del ADN plasmídico (véase el diagrama “Procedimiento de QIAGEN Plasmid Kit”). El ADN eluido se mezcla con isopropanol y se aplica al módulo QIAprecipitator utilizando la jeringa suministrada. A continuación, el ADN concentrado y desalado se eluye del QIAprecipitator directamente en un tubo de microcentrifugadora con tampón TE o agua.
Aplicaciones
El ADN purificado con los HiSpeed Plasmid Kits da excelentes resultados en todas las aplicaciones, desde la clonación y la secuenciación, hasta la transfección y el silenciamiento génico mediado por plásmidos.
Datos y cifras de respaldo
Módulo QIAprecipitator.