✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAamp 96 Virus QIAcube HT Kit (5)
Cat. No. / ID: 57731
For 480 preps: QIAamp 96 plates, QIAGEN Proteinase K, carrier RNA, buffers
KitAccessories
QIAamp 96 Virus QIAcube HT Kit
QIAcube HT Plasticware
The QIAamp 96 Virus QIAcube HT Kit is intended for molecular biology applications. This product is not intended for the diagnosis, prevention, or treatment of a disease.
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Simple and reliable automated processing for cost and time savings
- Suitable for many samples, including blood, tissues, swabs and body fluids
- Consistent, high yields
- Efficient removal of inhibitors and contaminants
- Purified nucleic acids ready for analysis by real-time PCR or RT-PCR
Product Details
The QIAamp 96 Virus QIAcube HT Kit enables simple, automated purification of viral RNA and DNA on the QIAcube HT system. Using proven QIAamp silica-membrane technology in a convenient 96-well format, contaminants and inhibitors are removed to yield high-quality nucleic acids that are ready for downstream analysis.
Performance
The QIAamp 96 Virus QIAcube HT Kit enables automated purification of viral RNA and DNA from a broad range of sample types including fresh or frozen tissues, blood and other body fluids. The procedure yields high-quality viral nucleic acids that perform well in downstream PCR and RT-PCR analyses (see figure Reliable purification from a range of sample types).
QIAcube HT with the dedicated QIAamp 96 Virus QIAcube HT Kit lets users increase sample purification throughput without having to compromise on quality or reliability. The procedure provides high yields of pure RNA or DNA that perform well in downstream analyses, similar to other QIAGEN DNA purification solutions (see figures Reliable automated purification and High sensitivity of viral nucleic acids in PCR and RT-PCR).
QIAcube HT with the dedicated QIAamp 96 Virus QIAcube HT Kit lets users increase sample purification throughput without having to compromise on quality or reliability. The procedure provides high yields of pure RNA or DNA that perform well in downstream analyses, similar to other QIAGEN DNA purification solutions (see figures Reliable automated purification and High sensitivity of viral nucleic acids in PCR and RT-PCR).
See figures
Principle
The QIAamp 96 Virus QIAcube HT Kit combines the selective binding properties of a silica-based membrane with a high-throughput 96-well format, and is designed for fully automated, simultaneous processing of 24–96 samples on the QIAcube HT instrument.
| Specification | Description |
|---|---|
| Number of samples | 24–96 samples (to be processed in increments of 8) |
| Sample input volume | Blood and other body fluids: up to 200 μl (for sample volumes less than 200 μl, add PBS) Tissues: up to 20 mg tissue |
| Elution volume | 100 μl |
| Duration | 96 samples in approximately 145 minutes 24 samples in approximately 90 minutes |
Procedure
The QIAamp 96 Virus QIAcube HT procedure is fast and simple with the QIAcube HT instrument. Samples are lysed under highly denaturing conditions at room temperature in the presence of QIAGEN proteinase K and Buffer ACL, which together ensure the inactivation of nucleases. Adding Buffer ACB adjusts the binding conditions for the co-purification of DNA and RNA. The lysate is then transferred to a QIAamp 96 plate. During vacuum, nucleic acids are adsorbed onto the silica membranes while contaminants pass through. Three efficient wash steps remove the remaining contaminants and enzyme inhibitors, and nucleic acids are eluted in Buffer AVE. Some samples may require a pretreatment.
Applications
The high-quality nucleic acids are ready to use in all downstream applications, including sensitive detection assays using quantitative, real-time PCR or RT-PCR.
Supporting data and figures
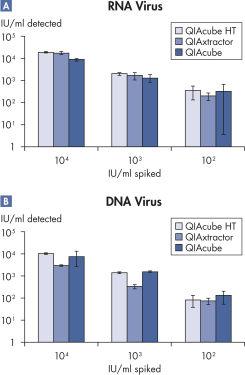
Reliable automated purification.
Viral nucleic acids were purified from 200 µl plasma samples spiked with 10,000, 1000 and 100 IU/ml of a typical DNA or RNA virus. Sample processing was automated using either QIAcube HT with the QIAamp 96 Virus QIAcube HT Kit and protocol, QIAxtractor with the VX Protocol, or QIAcube with the QIAamp MinElute Virus Spin Kit. Viral nucleic acids were detected using in-house PCR and RT-PCR assays, with 20 µl eluate per reaction on the Rotor-Gene Q. Results show that QIAcube HT with the QIAamp 96 Virus QIAcube HT Kit performs as well as or better than the other methods.
Resources
Quick-Start Protocols (3)
Protocol Files (2)
Safety Data Sheets (1)
Technical Information (2)
Kit Handbooks (2)
Instrument User Manuals (3)
Brochures & Guides (1)
Certificates of Analysis (1)
FAQ
Can I run the QIAcube HT with the QIAxtractor software or vice versa?
For what batch size is the QIAcube HT Plasticware designed?
Can I upgrade a Corbett CAS-1820 to a QIAcube HT?
What file is required for reporting a QIAcube HT or QIAxtractor issue?
I am getting vacuum errors on my QIAcube HT. What should I do?
What do I do if the QIAcube HT or QIAxtractor software runs in virtual mode while the instrument is connected to the computer?