✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 33903
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Keine zeitaufwändigen Subklonierungsverfahren erforderlich
- Erhalten Sie posttranslationale Modifikationen in Insekten- und Säugerzellen
- Ein Konstrukt für effiziente Expression in drei Expressionssystemen
Product Details
Performance
See figures
Principle
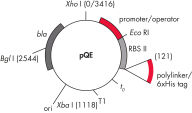
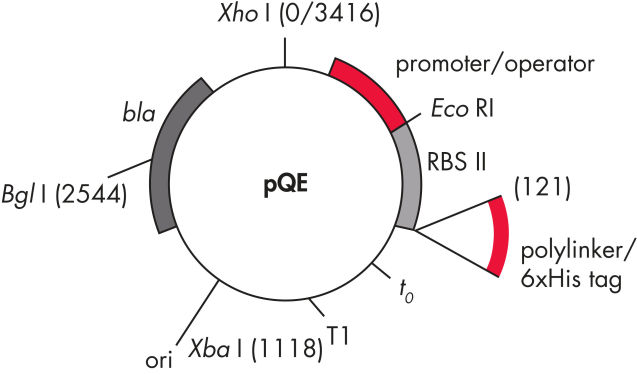
QIAexpress pQE-Vektoren kombinieren einen leistungsstarken T5-Phagen-Promotor (der von der E. coli-RNA-Polymerase erkannt wird) mit einem doppelten lac-Operator-Repressionsmodul, um streng regulierte, hohe Expressionslevel rekombinanter Proteine in E. coli zu ermöglichen. Die Proteinsynthese wird in Gegenwart großer Mengen des lac-Repressors wirksam blockiert und die Stabilität der zytotoxischen Konstrukte erhöht. Die pQE-Vektoren (siehe Tabelle und Abbildung pQE-Vektoren) ermöglichen die Positionierung des 6xHis-tags entweder am N- oder C-Terminus des rekombinanten Proteins.
| Element | Beschreibung |
| 1. Optimiertes Promotor-/Operatorelement |
Besteht aus dem T5-Phagen-Promotor und zwei lac-Operatorsequenzen, die die Bindungswahrscheinlichkeit des lac-Repressors erhöhen und eine effiziente Unterdrückung des starken T5-Promotors gewährleisten |
| 2. Synthetische ribosomale Bindungsstelle RBSII | Für effiziente Translation |
| 3. His-tag-kodierende Sequenz | Entweder 5' oder 3' von der Polylinker-Klonierregion |
| 4. Translations-Stoppcodons | In allen Leserahmen zur einfachen Herstellung von Expressionskonstrukten |
| 5. Zwei starke Transkriptionsterminatoren |
t0 vom Lambda-Phagen und T1 vom rrnB-Operon von E. coli, um eine Read-Through-Transkription zu verhindern und die Stabilität des Expressionskonstrukts zu gewährleisten |
|
6. ColE1-Replikationsursprung |
Aus pBR322 |
| 7. Beta-Lactamase-Gen (bla) | Verleiht Ampicillin-Resistenz |
See figures
Procedure
Inserts, die für die untersuchten Proteine kodieren, werden in geeignete Konstrukte kloniert und zur Expression in einen geeigneten E. coli-Stamm transformiert. Die Expression wird durch Zugabe von IPTG induziert. pQE-TriSystem Vector-Konstrukte können in E. coli transformiert, als Shuttle-Vektor für die rekombinante Proteinexpression in Insektenzellen verwendet oder in Säugerzellen transfiziert werden.
Applications
Das QIAexpress Expressionssystem ermöglicht hohe Expressionslevel von Proteinen, die sich
für viele Anwendungen eignen wie:
- Aufreinigung funktioneller Proteine mit aktiver Konformation
- Aufreinigung unter denaturierenden Bedingungen für die Antikörperproduktion
- Kristallisierung zur Bestimmung der dreidimensionalen Struktur
- Protein-Protein- und Protein-DNA-Interaktionsassays
Supporting data and figures
pQE-Vektoren.
In der Tabelle aufgeführte nummerierte Elemente.
Specifications
| Features | Specifications |
|---|---|
| In-frame cloning necessary | Ja |
| Expression | In vivo |
| Tag removal sequence | Nein |
| Expression species | E. coli, Säugerzellen, Insektenzellen |
| Tag | 6xHis-tag |
| N- or C-terminal tag | C-terminaler Tag |
| All three reading frames provided | Nein |