Products
Features
- High-performance assays created with a convenient design tool and the same algorithm used for our predesigned assays
- Custom assays to match your exact target and need, including detection of novel transcripts or a specific isoform or splice variant
- Ability to submit up to 10 sequences and design assays to differentiate or detect all sequences
- Short, LNA-enhanced primers with high specificity and greater flexibility in assay positioning
- Absolute quantification of expression changes with dPCR using the QIAcuity instrument and the QIAcuity EG PCR Kit
Product Details
QuantiNova LNA PCR Custom Assays provide accurate and sensitive gene expression analysis of any RNA target using LNA-enhanced, EvaGreen-based digital PCR. Our robust design algorithm provides full Ensembl database support for human, mouse and rat targets and streamlines assay creation. Custom assays are well-suited for targets for which no predesigned assay is available, for discriminating splice variants and for verifying novel mRNAs or lncRNAs. For digital PCR, we have optimized the assays with the QIAcuity EG PCR Kits and the QIAcuity instrument and created a simple and fast absolute quantification workflow that takes only 2 hours.
Are you planning to use the QuantiNova LNA PCR Custom Assays for qPCR analysis? You can find qPCR-specific product and performance details on the dedicated qPCR catalog page.
Need a quote for your research project or would you like to discuss your project with our specialist team? Just contact us!
Performance
Optimized digital PCR analysis with the QIAcuity EG PCR Kits and the QIAcuity instrument
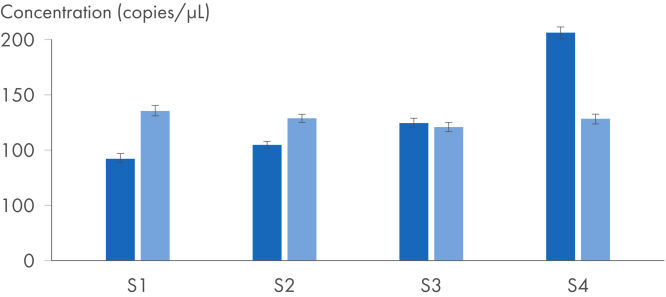
QuantiNova LNA PCR Assays were developed to provide high-specificity target amplification. This can be quantified by two PCR methods: qPCR with standard real-time thermocyclers and digital PCR using the QIAcuity instrument and the QIAcuity EG PCR Kit (see figure Using QuantiNova LNA PCR Assays for digital PCR). After cDNA synthesis using the QuantiTect Reverse Transcription Kit, digital PCR provides highly precise target quantification, detecting even the smallest expression changes at the lowest concentrations. A subset of key assays have been experimentally validated on the QIAcuity instrument.
Highly sensitive and specific LNA-enhanced assays
QuantiNova LNA PCR Custom Assays are created using stringent design criteria and lab-validated algorithms to deliver the highest specificity and efficiency for reliable and accurate gene expression analysis results.
The high binding affinity of LNA bases increases the flexibility of primer placement on the transcript, so we can use intelligent positioning to design assays for otherwise difficult-to-analyze targets. This gives you better target discrimination and robust and reliable quantification, even for AU-rich targets, low-abundance transcripts, targets with high secondary structure and highly complex samples.
The assays’ high sensitivity ensures excellent amplification efficiencies down to 1 RNA molecule, making it easier for you to detect low-abundance targets such as lncRNAs from less starting material. Increased specificity from the clever placement of LNA provides a high signal-to-noise ratio, allowing discrimination of sequences that differ by only a single nucleotide and eliminating non-specific amplification and primer-dimer formation.
Created using a sophisticated LNA design algorithm
Twenty years of LNA design experience has enabled us to develop and optimize our sophisticated LNA design algorithm, which incorporates over 50 different parameters – all thoroughly lab-validated against highly stringent performance criteria – to guarantee the most optimal assay for successful target detection. This proprietary algorithm has been used to design over 1.3 million assays and is available for your specialized requests through our convenient custom assay builder in GeneGlobe.
Principle
EvaGreen-based digital PCR
For absolute quantification by dPCR, use the QuantiNova LNA PCR Assays together with the QIAcuity EG PCR Kit, which uses EvaGreen-based fluorescence detection. EvaGreen is an intercalating dye that binds to double-stranded DNA (similarly to SYBR® Green) and fluoresces upon DNA binding. Using EvaGreen-based dPCR provides convenience and savings, because you only need the primer set to amplify and detect the product. It also provides higher resolution in the dPCR reaction, which is divided into thousands of partitions of the dPCR nanoplate, so the primer concentration needed for the dPCR reaction is only half that required for qPCR.
The custom design process
The QuantiNova LNA PCR Custom Assay design tool lets you easily design highly sensitive and specific, LNA-enhanced PCR assays for any mRNA or lncRNA not available as a predesigned assay. Using the advanced QuantiNova PCR Assay design algorithm, numerous assay combinations are evaluated based on more than 50 different criteria to find the optimal assay for your target within a few minutes. The tool has been designed for human, mouse and rat mRNA and lncRNA targets of at least 55 nucleotides and blasts against the species-relevant databases, including Ensembl and RefSeq. For known gene targets, assays are designed as intron-spanning by default, similar to the predesigned assays, to prevent the risk of gDNA amplification.
Uniquely advanced assay design options
The custom assay design tool includes options to create transcript-specific designs for discriminating splice variants, SNPs or isoforms. Alternatively, designs for assays common to all transcript variants can also be created. You can submit up to 10 different target sequences at once and design either ten different assays, or for related transcripts, you can design a common assay for all.
Reference Gene Assays can be easily combined with the custom assays
A wide selection of functionally validated human, mouse and rat Reference Gene Assays are available to enable high-quality data normalization and ensure reliable results. These assays target endogenous coding RNAs, long non-coding RNA and small nucleolar RNA molecules that are typically constitutively expressed in a wide variety of tissues.
Normalization of mRNA/lncRNA qPCR results
Normalization removes technical and biological inter-sample variation unrelated to the biological changes under investigation. Proper normalization is critical for correct analysis and interpretation of results. Most commonly, stably expressed reference genes are used for normalization.
It is generally recommended to test several endogenous control reference gene candidates before setting up your actual mRNA/lncRNA expression analysis. These candidates should be chosen from genes expected to be stably expressed over the whole range of samples under investigation. They could be stably expressed mRNAs or lncRNAs selected based on literature or preexisting data (e.g., NGS or qPCR panel screening). The QuantiNova LNA PCR system offers validated reference gene assays for RNAs that tend to be stably expressed and are therefore good candidates as reference genes.
All reference gene candidates should be empirically validated for each study. One option for normalizing PCR panel when profiling a large number of mRNAs/lncRNAs is to normalize against the global mean – the average of all expressed mRNAs/lncRNAs. This can be a good option in samples with a high call rate (expressed genes) but should be used with caution in samples with low call rates. It is also not a good option in samples for which the general gene expression level is changed. Further guidance on normalization can also be found in the GeneGlobe Data Analysis Center.
Digital PCR data analysis
The QIAcuity Software Suite is used for analyzing the dPCR data and includes a gene expression test, which provides your results as fold change and fold regulation with publication-ready figures.
Procedure
Two-step RT-dPCR
The best results are obtained when performing the reverse transcription reaction with the QuantiTect Reverse Transcription Kit (cat. nos, 205311, 205313, 205314). It is not recommended to use the QuantiNova Reverse Transcription Kit. The resulting cDNA is then quantified by dPCR using the master mixes of the QIAcuity EG PCR Kit combined with your choice of QuantiNova LNA PCR Assay.
Important note: The reaction amounts indicated in parentheses after the assay product names are relevant for qPCR use. Digital PCR only requires half the primer concentration, but reaction volumes are different. Using the QIAcuity Nanoplate 26K you can set up the same numbers of reactions as indicated; using the Nanoplate 8.5K you can set up 3.3x as many. Please refer to the handbook for QIAcuity use for dPCR instructions.
Shipping and delivery
QuantiNova LNA PCR Assays are shipped at ambient temperatures. In-stock assays are delivered within 1–5 days. During the early access period, longer delivery times should be expected.
What you need to get started for digital PCR with the QuantiNova LNA PCR system
Reverse transcription: QuantiTect Reverse Transcription Kit (cat. nos, 205311, 205313, 205314)
dPCR mastermix: QIAcuity EG PCR Kit
Assays:
Applications
QuantiNova LNA PCR Custom Assays are highly suited for applications including:
- mRNA and lncRNA expression analysis, profiling and quantification
- Validation of RNA-seq gene expression data
- Gene expression profiling
- Signal and pathway analysis
- Confirming gene expression knockdown by LNA GapmeRs or siRNAs
- Biomarker development, including screening, identification and validation of disease-associated biomarkers
- Monitoring phenotypic changes related to gene expression
Multiple QuantiNova LNA PCR Assays can also be used to examine a focused panel of genes.
Supporting data and figures
IL-4 gene expression analysis – detecting small expression changes with the highest precision.