✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Results in 3–20 minutes
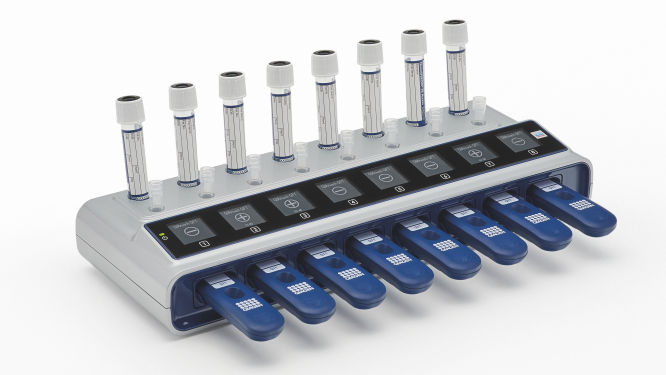
- Test up to 8 patients simultaneously on each eHub
- Walkaway and random access
- Digital and connected
Product Details
The QIAreach QuantiFERON-TB (QIAreach QFT) assay is an in vitro diagnostic test using a peptide cocktail simulating ESAT-6 and CFP-10 proteins to stimulate cells in heparinized whole blood. Detection of interferon gamma (IFN-γ) by nanoparticle fluorescence is used to identify in vitro responses to these peptide antigens that are associated with Mycobacterium tuberculosis infection.
QIAreach QFT is a semi-automated, indirect test for M. tuberculosis infection (including disease) and is intended for use in conjunction with risk assessment, radiography, and other medical and diagnostic evaluations.
QIAreach QFT is an indirect test to screen for M. tuberculosis infection (including disease) and is intended for use in at-risk populations. There are no known population restrictions for the use of QIAreach QFT.
Performance
The clinical performance study for QIAreach QFT compared the clinical accuracy (concordance) between the QIAreach QFT system and well-established internationally recognized reference TB infection diagnostic method QuantiFERON-TB Gold Plus (QFT-Plus). QFT-Plus is CE-IVD marked and FDA approved. A total of 225 subjects were tested with both the QFT-Plus ELISA reference method and the QIAreach QFT system, made up of 150 QFT-Plus negative subjects and 75 QFT-Plus positive subjects.
Clinical agreement
The clinical agreement levels of QIAreach QFT Positive and Negative results with QFT-Plus Positive and Negative results are reported in Table 1.
Table 1. Clinical Agreement: QIAreach QFT result vs. QFT-Plus result (reference)
| QIAreach QFT | QFT-Plus | ||
| Negative (-) | Positive (+) | Total | |
|---|---|---|---|
| Negative (-) | 148 | 4 | 152 |
| Positive (+) | 2 | 71 | 73 |
| Total | 150 | 75 | 225 |
The positive percent agreement (PPA), negative percent agreement (NPA), and overall percent agreement (OPA) between the results of QIAreach QFT and QFT-Plus as the reference method were as follows (Table 2):
Table 2. QIAreach QFT versus QFT-Plus
| Frequency | Agreement | Upper 95% CI | Lower 95% CI | |
|---|---|---|---|---|
| OPA* | 219/225 | 97.3% | 99.0% | 94.3% |
| PPA | 71/75 | 94.7% | 98.5% | 86.9% |
| NPA | 148/150 | 98.7% | 99.8% | 95.3% |
OPA: Overall percent agreement; PPA: Positive percent agreement; NPA: Negative percent agreement
* When factoring in 15 QFT-Plus indeterminate results, the OPA between QFT-Plus and QIAreach QFT is 91.3% (95% CI: 86.9 – 94.5%).
Principle
The QIAreach QFT assay uses a specialized Blood Collection Tube, which is used to collect whole blood. Incubation of the blood occurs in the tube for 16–24 hours, after which, plasma is harvested and tested for the presence of IFN-γ produced in response to the peptide antigens.
The QIAreach QFT test is performed in two stages. First, whole blood is collected into a QIAreach QFT Blood Collection Tube.
The QIAreach QFT Blood Collection Tube is mixed and should be incubated at 37°C as soon as possible, and within 16 hours of collection. Following a 16–24 hour incubation period, the tube is centrifuged, the plasma is removed and mixed in a sample Processing Tube and the amount of IFN-γ is measured in a cartridge integrated with digital detection.
To perform the detection assay, QIAreach QFT Diluent Buffer is first added to the Processing Tube and reconstitutes an anti-IFN-γ antibody-nanoparticle conjugate that is spray-dried on an immobilized accretion pad within the tube. Plasma is removed from the QIAreach QFT Blood Collection Tube and added to the Processing Tube and mixed with the resuspended conjugate. If IFN-γ is present in the sample, it will bind to the conjugate. The sample is then transferred from the Processing Tube to the eStick sample port.
Once in the eStick, the test sample migrates on a nitrocellulose membrane and across the test line. The IFN-γ antibody-nanoparticle conjugate will bind to immobilized anti-IFN-γ capture antibody at the test line. A photosensor will detect light emitted from the fluorescent nanoparticles in the presence of excitation light filtered onto the test line. Signal is interpreted on the eStick firmware and transmitted to the eHub, which then communicates a positive or negative test result to the user by means of a visual display.
A QIAreach QFT test result with an IFN-γ response that is above the signal threshold is considered positive for MTB infection. IFN-γ responses below this threshold are considered negative for MTB infection.