Ni-NTA Agarose
For purification of His-tagged proteins by gravity-flow chromatography
For purification of His-tagged proteins by gravity-flow chromatography
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 30210
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Ni-NTA Agarose is an affinity chromatography matrix for purifying recombinant proteins carrying a His tag. Histidine residues in the His tag bind to the vacant positions in the coordination sphere of the immobilized nickel ions with high specificity and affinity. Cleared cell lysates are loaded onto the matrices. His-tagged proteins are bound, and other proteins pass through the matrix. After washing, His-tagged proteins are eluted in buffer under native or denaturing conditions
Ni-NTA Agarose provides Ni-NTA coupled to a Sepharose CL-6B support and offers high binding capacity and minimal nonspecific binding (see figure One-step purification under native conditions). This material has excellent handling properties for most scales of batch and column purification. Ni-NTA Agarose is available separately or as a component of QIAexpress Kits, which are complete kits for efficient expression and purification of His-tagged proteins from E. coli .
The QIAexpress Ni-NTA Protein Purification System is based on the remarkable selectivity of patented Ni-NTA (nickel-nitrilotriacetic acid) resin for proteins which contain an affinity tag of six or more histidine residues (consecutive or alternating) — the His tag. This technology allows one-step purification of almost any His-tagged protein from any expression system under native or denaturing conditions. NTA, which has four chelation sites for nickel ions, binds nickel more tightly than metal-chelating purification systems that only have three sites available for interaction with metal ions. The extra chelation site prevents nickel-ion leaching and results in a greater binding capacity and protein preparations with higher purity than those obtained using other metal-chelating purification systems. The QIAexpress system can be used to purify His-tagged proteins from any expression system, including baculovirus, mammalian cells, yeast, and bacteria.
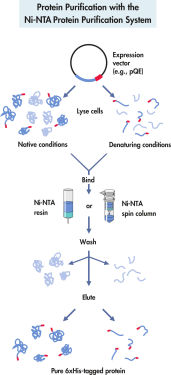
The purification of His-tagged proteins consists of 4 stages: cell lysis, binding, washing, and elution (see Protein purification with the Ni-NTA protein purification system). Purification of recombinant proteins using the QIAexpress system does not depend on the 3-dimensional structure of the protein or 6xHis tag. This allows one-step protein purification under either native or denaturing conditions, from dilute solutions and crude lysates. Strong denaturants and detergents can be used for efficient solubilization and purification of receptors, membrane proteins, and proteins that form inclusion bodies. Reagents that allow efficient removal of nonspecifically binding contaminants can be included in wash buffers (see table). Purified proteins are eluted under mild conditions by adding 100–250 mM imidazole as competitor or by a reduction in pH.
| Denaturants | Detergents | Reducing agents | Others | Salts | For long-term storage |
|---|---|---|---|---|---|
| 6 M Gu·HCl | 2% Triton X-100 | 20 mM β-ME | 50% Glycerol | 4 M MgCl2 | Up to 30% ethanol |
| 8 M Urea | 2% Tween 20 | 10 mM DTT | 20% Ethanol | 5 mM CaCl2 | or 100 mM NaOH |
| 1% CHAPS | 20 mM TCEP | 20 mM Imidazole | 2 M NaCl |
Ni-NTA matrices provide reliable, one-step purification of His-tagged proteins suitable for any application, including:
| Features | Specifications |
|---|---|
| Applications | Proteomics |
| Tag | 6xHis tag |
| Bead size | 45–165 µm |
| Special feature | Batch and column purification |
| FPLC | No |
| Processing | Manual |
| Support/matrix | Sepharose CL-6B |
| Start material | Cell lysate |
| Scale | Large scale |
| Binding capacity | Up to 50 mg/ml |
| Gravity flow or spin column | Gravity flow |
| Yield | 100 µg – 100 mg |